ACT Exam > ACT Questions > Directions: Read the passage and choose the b...
Start Learning for Free
Directions: Read the passage and choose the best answer to each question.
Passage
Researchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.
Study 1
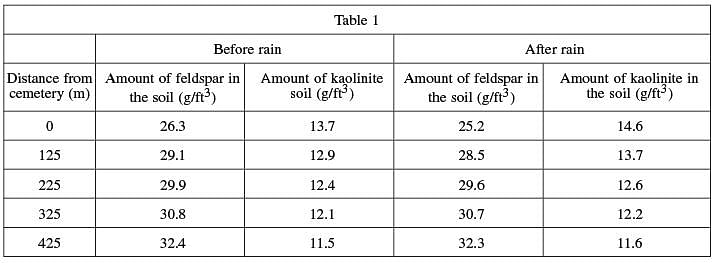
A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and other locations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)
Study 2
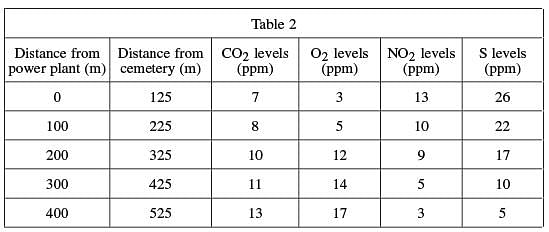
The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.
Passage
Researchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.
Study 1
A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and other locations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)
Study 2
The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.


Q. According to the results of Study 2, as distance from the power plant decreases:
- a)sulfur levels decrease.
- b)sulfur and NO2 levels increase.
- c)NO2 and O2 levels decrease.
- d)CO2 and O2 levels increase.
Correct answer is option 'B'. Can you explain this answer?
Most Upvoted Answer
Directions: Read the passage and choose the best answer to each questi...
Table 2 shows that as the distance from the power plant decreases (moving up the table), the levels of sulfur and NO2 increase.
Answer choice C can be eliminated because NO2 and O2 do not behave similarly.
Answer choice C can be eliminated because NO2 and O2 do not behave similarly.

|
Explore Courses for ACT exam
|

|
Similar ACT Doubts
Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer?
Question Description
Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer?.
Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer?.
Solutions for Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions: Read the passage and choose the best answer to each question.PassageResearchers have noticed sudden decay in limestone grave markers in a cemetery downwind from a coal-burning power plant. Two studies were conducted to examine this decay process.Study 1A key mineral component of limestone is feldspar, which is also abundant in ground soil. Feldspar, as it weathers and decays, breaks down into another mineral, kaolinite. The weathering process is often expedited by moisture from rain and humidity. Researchers measured the levels of feldspar and kaolinite in the soil of the cemetery and otherlocations at specific distances from the cemetery before and after rain showers. The results are shown in Table 1. (Note: The soil sample sizes were the same for each location tested.)Study 2The researchers believe that the emissions from the nearby power plant are somehow affecting the decay of the limestone. Levels of common gases – carbon dioxide (CO2), oxygen (O2), nitrogen dioxide (NO2), and sulfur (S) – in the atmosphere were tested at the cemetery and other locations at specific distances from both the cemetery and the power plant. Measurements in parts per million (ppm) were recorded in Table 2.Q.According to the results of Study 2, as distance from the power plant decreases:a)sulfur levels decrease.b)sulfur and NO2 levels increase.c)NO2 and O2 levels decrease.d)CO2 and O2 levels increase.Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























