ACT Exam > ACT Questions > Directions:Read the passage and choose the be...
Start Learning for Free
Directions: Read the passage and choose the best answer to each question.
Passage
People use many different chemicals each day for common household tasks such as cleaning and food preparation.
Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.
Experiment 1
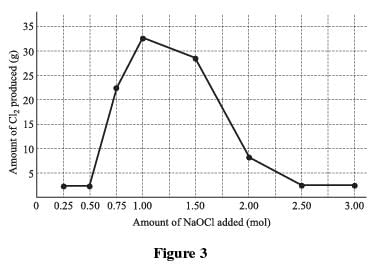
A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.
Experiment 2
Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.
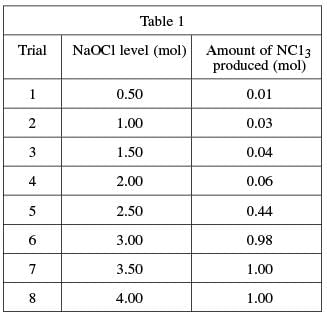
To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity added was gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.
Experiment 3
In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.
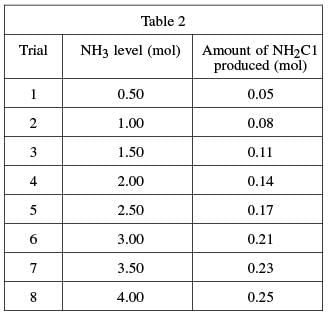
To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.
Passage
People use many different chemicals each day for common household tasks such as cleaning and food preparation.
Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.
Experiment 1
A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.

Experiment 2
Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.
To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity added was gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.

Experiment 3
In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.
To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.

Q. Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:
- a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.
- b)when more bleach was added the mixture became too volatile.
- c)adding more bleach no longer increased the level of ammonia.
- d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.
Correct answer is option 'D'. Can you explain this answer?
Most Upvoted Answer
Directions:Read the passage and choose the best answer to each questio...
The graph shows the chlorine gas levels decreasing after 1.00 mole of bleach was added. Because the chlorine levels continued to decrease as bleach was added, it was unnecessary to proceed further. The other answer choices are not supported by the data.

|
Explore Courses for ACT exam
|

|
Similar ACT Doubts
Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer?
Question Description
Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer?.
Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer?.
Solutions for Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer?, a detailed solution for Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions:Read the passage and choose the best answer to each question.PassagePeople use many different chemicals each day for common household tasks such as cleaning and food preparation.Since the inception of consumer protection laws, chemicals come with toxicity warning labels, directions about proper use, and cautions about the hazards of improper use. Some household chemicals can be quite dangerous, especially when mixed together. One such example is the reaction that occurs when mixing household bleach (NaOCl) with ammonia (NH3). The by-products of the reaction vary depending on the concentrations of the reactants. The following experiments were conducted to determine the levels at which certain by-products resulted from mixing bleach and ammonia.Experiment 1A known by-product of the reaction of bleach and ammonia is chlorine gas (Cl2). Chlorine gas has an intensely disagreeable suffocating odor, and is very poisonous. To determine the quantities of bleach and ammonia that, when mixed together, produce chlorine gas, a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each of the solutions; the amount added was gradually increased for each trial. The amount of chlorine gas produced in each trial was recorded and graphed in Figure 1.Experiment 2Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogen containing compounds and chlorine. It is highly explosive.To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity addedwas gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.Experiment 3In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination.To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.Q.Which of the following is the most likely reason that amounts greater than 3.00 mol of bleach were not tested in Experiment 1? The results showed that:a)amounts less than 3.00 mol of bleach increased the amount of chlorine gas produced.b)when more bleach was added the mixture became too volatile.c)adding more bleach no longer increased the level of ammonia.d)amounts greater than 1.00 mol of bleach decreased the amount of chlorine gas produced.Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























