ACT Exam > ACT Questions > Directions:Read the passages and choose the b...
Start Learning for Free
Directions: Read the passages and choose the best answer to each question.
Passage
Radioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.
This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.
Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.
Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.
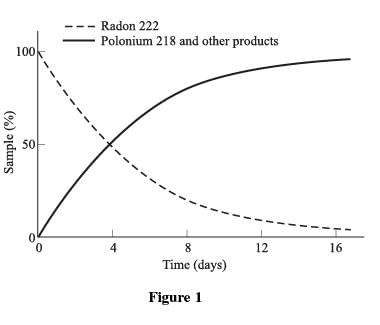
Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.
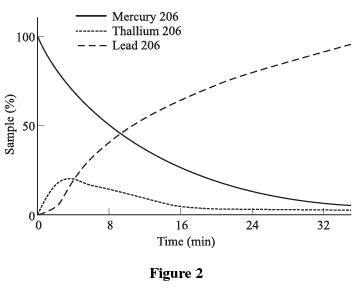
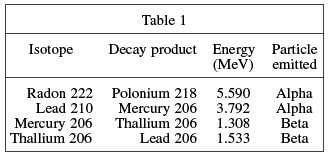
Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.
Passage
Radioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.
This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.
Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.
Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.

Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.

Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.

Q. Based on Table 1, what is the relationship between decay energy and the type of particle emitted?
- a)Beta particles tend to have higher decay energies.
- b)Alpha particles tend to have lower decay energies.
- c)Alpha particles tend to have higher decay energies.
- d)There is no apparent relationship between type of particle and decay energy.
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
Directions:Read the passages and choose the best answer to each questi...
According to Table 1, the energies associated with alpha particle emission were 5.590 MeV and 3.792 MeV. The energies associated with beta particle emission were 1.308 MeV and 1.533 MeV—lower than that of the alpha particle emission.

|
Explore Courses for ACT exam
|

|
Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer?
Question Description
Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer?.
Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer?.
Solutions for Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions:Read the passages and choose the best answer to each question.PassageRadioactive decay is a natural process by which an atom of a radioactive isotope (chemical element) spontaneously decays into another element. The unstable nucleus disintegrates by emitting alpha or beta particles, or gamma rays.This process changes the composition of the nucleus and continues to take place until a stable nucleus is reached.Half-life refers to the amount of time it takes for half (50%) of the atoms in a sample to decay.Figure 1 shows the decay from Radon 222 to Polonium 218 and other decay products.Figure 2 shows the decay from Mercury 206 to Thallium 206 to Lead 206.Table 1 shows decay products and associated energy in MeV, million electron volts, and the type of particle emitted from the decay.Q.Based on Table 1, what is the relationship between decay energy and the type of particle emitted?a)Beta particles tend to have higher decay energies.b)Alpha particles tend to have lower decay energies.c)Alpha particles tend to have higher decay energies.d)There is no apparent relationship between type of particle and decay energy.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























