ACT Exam > ACT Questions > Directions:Read the passages and choose the b...
Start Learning for Free
Directions: Read the passages and choose the best answer to each question.
Passage
When connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72◦ F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.
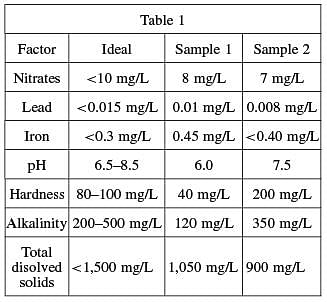
The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.
The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.
Water with a pH > 8.5 could indicate that the water is hard.
Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.
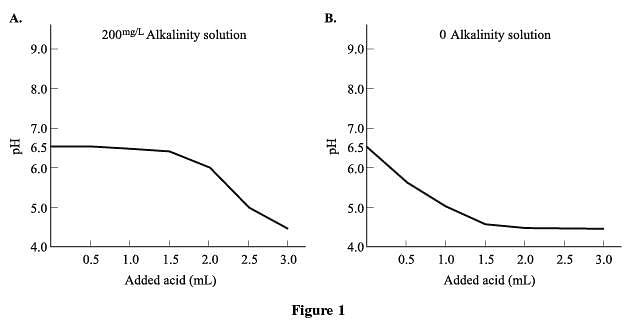
Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.
Passage
When connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72◦ F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.

The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.
The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.
Water with a pH > 8.5 could indicate that the water is hard.
Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.
Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.

Q. An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:
- a)an alkalinity test is not necessary.
- b)an alkalinity level above 500 is ideal.
- c)ideal samples will have levels similar to that of Sample 1.
- d)a proper alkalinity level can prevent water from becoming overly corrosive.
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Directions:Read the passages and choose the best answer to each questi...
The passage explains, “Water with a low pH (<6.5) could be acidic, soft, and corrosive.” If an ideal alkalinity level prevents pH levels from becoming too low, it will also prevent water from becoming overly corrosive.
Answer choice B can be eliminated because the ideal alkalinity level shown in Table 1 is between 200 and 500 mg/L.
Answer choice B can be eliminated because the ideal alkalinity level shown in Table 1 is between 200 and 500 mg/L.
Most Upvoted Answer
Directions:Read the passages and choose the best answer to each questi...
The passage explains, “Water with a low pH (<6.5) could be acidic, soft, and corrosive.” If an ideal alkalinity level prevents pH levels from becoming too low, it will also prevent water from becoming overly corrosive.
Answer choice B can be eliminated because the ideal alkalinity level shown in Table 1 is between 200 and 500 mg/L.
Answer choice B can be eliminated because the ideal alkalinity level shown in Table 1 is between 200 and 500 mg/L.

|
Explore Courses for ACT exam
|

|
Similar ACT Doubts
Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer?
Question Description
Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer?.
Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer?.
Solutions for Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions:Read the passages and choose the best answer to each question.PassageWhen connection to a municipal water system is not feasible, wells are drilled to access ground water. Engineers employed by a company interested in developing a remote plot of land conducted studies to compare the water quality of 2 possible well locations on the land. Water quality is determined by a number of factors, including the levels of nitrates, lead, microbes, pH, “hardness” (calcium carbonate), and alkalinity. The water samples were kept at a constant temperature of 72 F throughout the study. The results in Table 1 show the readings of each test for the two different 100 mL samples of water, as well as the ideal level, or concentration, for each chemical.The pH scale measures how acidic or basic a substance is on a scale of 0 to 14. Lower numbers indicate increasing acidity and higher numbers indicate increasing basicity.The normal pH level of groundwater systems is between 6 and 8.5. Water with a low pH (<6.5) could be acidic, soft, and corrosive, and could contain elevated levels of toxic metal that might cause premature damage to metal piping.Water with a pH > 8.5 could indicate that the water is hard.Hard water does not pose a health risk, but can cause mineral deposits on fixtures and dishes and can have a bad taste and odor.Alkalinity is the water’s capacity to resist decreases in pH level. This resistance is achieved through a process called buffering (a buffered solution resists changes in pH until the buffer is used up). Alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks that contain carbonate, bicarbonate, and hydroxide compounds. These compounds, however, also cause hardness, which is less desirable in a drinking source. To illustrate the affect of alkalinity on pH stability, acid was added to two 100 milliliters sample solutions that initially had a pH of 6.5. The solution in Figure 1A had an alkalinity level of 200 mg/L while the solution in Figure 1B tested at zero alkalinity. The pH of the two solutions was recorded after every addition of acid and the results are shown in the figures below.Q.An ideal alkalinity level prevents pH levels from becoming too low. Which statement is best supported by this fact? When testing drinking water:a)an alkalinity test is not necessary.b)an alkalinity level above 500 is ideal.c)ideal samples will have levels similar to that of Sample 1.d)a proper alkalinity level can prevent water from becoming overly corrosive.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























