ACT Exam > ACT Questions > Directions: Read the passages and choose the ...
Start Learning for Free
Directions: Read the passages and choose the best answer to each question.
Passage
The photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.
The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.
Experiment 1
Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.
After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.
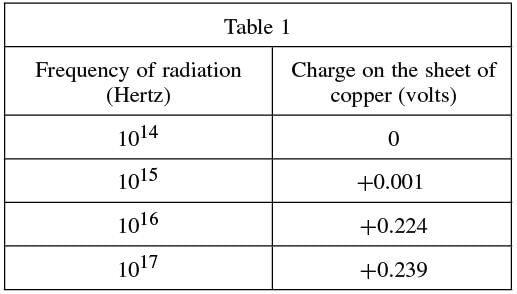
Passage
The photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.
The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.
Experiment 1
Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.
After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.

Experiment 2
Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.
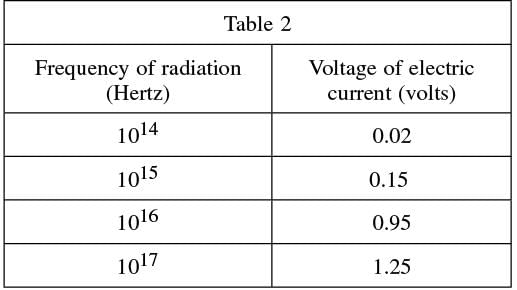
Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.

Q. Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?
- a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.
- b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.
- c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.
- d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Directions: Read the passages and choose the best answer to each quest...
The best answer is b. According to the results of Experiments 1 and 2, as the frequency of radiation increased, so did the electron emission (which required a high photon energy). This supports the definition of photons as finite packets of energy at various levels because higher frequency radiation caused the emission of electrons.

|
Explore Courses for ACT exam
|

|
Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer?
Question Description
Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer?.
Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer?.
Solutions for Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions: Read the passages and choose the best answer to each question.PassageThe photoelectric effect is the emission of electrons from matter upon the absorption of electromagnetic radiation, such as ultraviolet radiation or X-rays. Electromagnetic radiation is made up of photons, which can be considered finite packets of energy at various levels. Photons have properties attributed to both particles and waves. This phenomenon is known as the wave-particle duality.The photoelectric effect is especially noticeable when dealing with metals. When a metallic surface is exposed to electromagnetic radiation that is above the minimum energy threshold (which is specific to the type of surface and material), photons are absorbed and electrons are emitted. No electrons are emitted for radiation with energy frequencies below that of the threshold, as the electrons are unable to gain sufficient energy to overcome the attractive forces within the metal. A scientist wishing to measure the photoelectric effect so as to further understand the nature of photons conducted the following experiments.Experiment 1Wishing to measure the energy required to produce the photoelectric effect on a surface of a sheet of copper, the scientist directed a beam of radiation at different frequencies (energies)—measured in Hertz (Hz)—onto the surface.After 5 minutes, the charge—measured in volts (V)—of the sheet of metal was recorded. This was done because if electrons were emitted from the surface, the metal would take on a positive charge. The results were recorded in Table 1.Experiment 2Solar cells used to generate electricity are based on the concept of the photoelectric effect; however, the goal of the cell is to capture the emitted electron and create an electric current. The scientist measured the effects of different frequencies (in Hz) of radiation on the current (in V) generated by a certain solar cell. The results were recorded in Table 2.Q.Do the results of the experiments help to explain the nature of photons as finite packets of energy at various levels?a)Yes, because the experiments illustrate how solar panels can produce more electricity when exposed to higher frequencies of radiation.b)Yes, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) causes emission of electrons, which require a minimum energy to escape the surface of the metal.c)No, because the experiments illustrate how higher frequency radiation (photons with higher energy levels) does not cause increased emission of electrons.d)No, because there is no relation between the energy level of photons and the rate of photoelectric emission of electrons.Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























