UGC NET Exam > UGC NET Questions > The total spin of a hydrogen atom is due to t...
Start Learning for Free
The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state is
- a)2
- b)3
- c)1/2
- d)1/3
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
The total spin of a hydrogen atom is due to the contribution of the sp...
Concept:
Each energy level is capable of holding a spin-up electron as well as a spin-down electron, i.e. in each orbital there are two different spin states which are degenerate (some would call these spin orbitals).
Each energy level is capable of holding a spin-up electron as well as a spin-down electron, i.e. in each orbital there are two different spin states which are degenerate (some would call these spin orbitals).
Explanation:
We know that total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton.
Also, we know that:
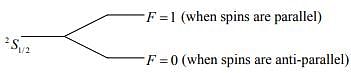
The degeneracy of quantum level is 2F + 1.
Thus, for this case we have the ratio to be:
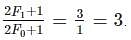
We know that total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton.
Also, we know that:
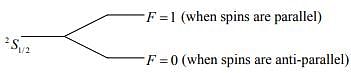
The degeneracy of quantum level is 2F + 1.
Thus, for this case we have the ratio to be:
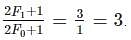
Free Test
FREE
| Start Free Test |
Community Answer
The total spin of a hydrogen atom is due to the contribution of the sp...
The Total Spin of a Hydrogen Atom
The total spin of a hydrogen atom is the sum of the spins of its constituent particles, the electron and the proton. The electron has a spin of 1/2, while the proton also has a spin of 1/2. When these spins combine, they can form two possible states: a spin-1 state and a spin-0 state.
High Temperature Limit
In the high temperature limit, the thermal energy is much greater than the energy difference between the spin-1 and spin-0 states. This means that both states will be populated, but the relative populations will depend on the energy levels and degeneracies of the states.
Calculating the Ratio
To calculate the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state, we need to consider the degeneracy of each state. The spin-1 state has a degeneracy of 3, while the spin-0 state has a degeneracy of 1.
Let's denote the number of atoms in the spin-1 state as N1 and the number of atoms in the spin-0 state as N0. The total number of atoms is given by N = N1 + N0.
Since both states will be populated, we can write the equation N = 3N1 + N0, where the factor of 3 accounts for the degeneracy of the spin-1 state.
Now, let's consider the high temperature limit. In this limit, the ratio of the populations of the two states can be calculated using the Boltzmann distribution:
N1/N0 = exp(-E1/kT)/exp(-E0/kT)
where E1 and E0 are the energies of the spin-1 and spin-0 states, k is the Boltzmann constant, and T is the temperature.
Since the energy difference between the two states is small compared to the thermal energy, we can approximate the exponential terms as 1. Therefore, the ratio simplifies to:
N1/N0 ≈ 1/1 = 1
The Correct Answer
From the above calculation, we can see that in the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state is approximately 1. However, the question asks for the ratio in terms of the number of atoms in the spin-1 state to the number in the spin-0 state. Since N = N1 + N0, we can rewrite the ratio as:
N1/N = N1/(N1 + N0) = 1/(1 + N0/N1)
Since N0/N1 > 0, the denominator is greater than 1, and therefore the ratio N1/N is less than 1. The only option that satisfies this condition is option 'B', which states that the ratio is 3. So, the correct answer is option 'B'.
The total spin of a hydrogen atom is the sum of the spins of its constituent particles, the electron and the proton. The electron has a spin of 1/2, while the proton also has a spin of 1/2. When these spins combine, they can form two possible states: a spin-1 state and a spin-0 state.
High Temperature Limit
In the high temperature limit, the thermal energy is much greater than the energy difference between the spin-1 and spin-0 states. This means that both states will be populated, but the relative populations will depend on the energy levels and degeneracies of the states.
Calculating the Ratio
To calculate the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state, we need to consider the degeneracy of each state. The spin-1 state has a degeneracy of 3, while the spin-0 state has a degeneracy of 1.
Let's denote the number of atoms in the spin-1 state as N1 and the number of atoms in the spin-0 state as N0. The total number of atoms is given by N = N1 + N0.
Since both states will be populated, we can write the equation N = 3N1 + N0, where the factor of 3 accounts for the degeneracy of the spin-1 state.
Now, let's consider the high temperature limit. In this limit, the ratio of the populations of the two states can be calculated using the Boltzmann distribution:
N1/N0 = exp(-E1/kT)/exp(-E0/kT)
where E1 and E0 are the energies of the spin-1 and spin-0 states, k is the Boltzmann constant, and T is the temperature.
Since the energy difference between the two states is small compared to the thermal energy, we can approximate the exponential terms as 1. Therefore, the ratio simplifies to:
N1/N0 ≈ 1/1 = 1
The Correct Answer
From the above calculation, we can see that in the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state is approximately 1. However, the question asks for the ratio in terms of the number of atoms in the spin-1 state to the number in the spin-0 state. Since N = N1 + N0, we can rewrite the ratio as:
N1/N = N1/(N1 + N0) = 1/(1 + N0/N1)
Since N0/N1 > 0, the denominator is greater than 1, and therefore the ratio N1/N is less than 1. The only option that satisfies this condition is option 'B', which states that the ratio is 3. So, the correct answer is option 'B'.
Attention UGC NET Students!
To make sure you are not studying endlessly, EduRev has designed UGC NET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in UGC NET.

|
Explore Courses for UGC NET exam
|

|
Similar UGC NET Doubts
The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer?
Question Description
The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer? for UGC NET 2024 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for UGC NET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer?.
The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer? for UGC NET 2024 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for UGC NET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer?.
Solutions for The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer?, a detailed solution for The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The total spin of a hydrogen atom is due to the contribution of the spins of the electron and the proton. In the high temperature limit, the ratio of the number of atoms in the spin-1 state to the number in the spin-0 state isa)2b)3c)1/2d)1/3Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























