UGC NET Exam > UGC NET Questions > Consider the following with regard to gas chr...
Start Learning for Free
Consider the following with regard to gas chromatography (GC).
A. Electron capture
B. Deemter equation
C. Partition Coefficient
D. Critical temperature of gas
Of the above, which are relevant to GC?
Choose the correct answer from the options given below:
A. Electron capture
B. Deemter equation
C. Partition Coefficient
D. Critical temperature of gas
Of the above, which are relevant to GC?
Choose the correct answer from the options given below:
- a)A, B and D only
- b)A, C and D only
- c)B, C and D only
- d)A, B and C only
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Consider the following with regard to gas chromatography (GC).A. Elect...
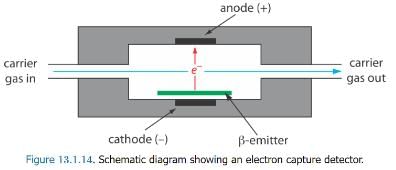
The electron capture detector is an example of a selective detector. Emitter, such as 63Ni. The emitted electrons ionize the mobile phase, usually N2, generating a standing current between a pair of electrodes. When a solute with a high affinity for capturing electrons elutes from the column, the current decreases, which serves as the signal. The ECD is highly selective toward solutes with electronegative functional groups, such as halogens and nitro groups, and is relatively insensitive to amines, alcohols, and hydrocarbons. Although its detection limit is excellent, its linear range extends over only about two orders of magnitude.

- The van Deemter equation is a hyperbolic function that predicts that there is an optimum velocity at which there will be the minimum variance per unit column length and, thence, a maximum efficiency. The van Deemter equation was the result of the first application of rate theory to the chromatography elution process.
- The most common mobile phases for gas chromatography are He, Ar, and N2, which have the advantage of being chemically inert toward both the sample and the stationary phase. The nature of the carrier gas has no significant influence on K, the partition coefficient, but it does have an effect on the solutes dispersion (has an effect on Neff and LOD). The choice of carrier gas often is determined by the needs of instrument detector. For a packed column the mobile phase velocity usually is 25–150 mL/min. The typical flow rate for a capillary column is 1–25 mL/min.
Thus, the correct option is 4.
Most Upvoted Answer
Consider the following with regard to gas chromatography (GC).A. Elect...
The correct answer is option 'D' - A, B and C only.
Let's discuss each option in detail and understand why they are relevant to gas chromatography (GC):
A. Electron Capture:
Electron capture is a detection technique used in gas chromatography. In this technique, a radioactive source emits beta particles, which are captured by the analyte molecules in the gas phase. This leads to the formation of negative ions, which can be detected and measured. Electron capture is particularly useful for the detection of electron-capturing compounds such as halogenated compounds, nitro compounds, and some pesticides.
B. Deemter Equation:
The Deemter equation is an empirical equation that describes the relationship between plate height (H) and linear flow velocity (u) in a chromatographic column. The equation is given as H = A + Bu + Cu^2, where A, B, and C are constants. The Deemter equation is used to optimize the separation efficiency of a chromatographic column by balancing the contributions of different factors such as longitudinal diffusion, resistance to mass transfer, and eddy diffusion.
C. Partition Coefficient:
Partition coefficient, also known as distribution coefficient, is a measure of the distribution of a solute between two immiscible phases, such as a stationary phase and a mobile phase in gas chromatography. It is defined as the ratio of the concentration of the solute in the stationary phase to the concentration of the solute in the mobile phase at equilibrium. The partition coefficient determines the degree of retention of a solute in the stationary phase, which affects its separation and elution time in gas chromatography.
D. Critical Temperature of Gas:
The critical temperature of a gas is the temperature above which the gas cannot be liquefied, regardless of the pressure. It is an important property to consider in gas chromatography because it determines the operating conditions for the separation. The critical temperature of a gas influences its behavior in the chromatographic column and affects factors such as diffusion, mass transfer, and retention time.
In conclusion, all the options A, B, and C - Electron Capture, Deemter Equation, and Partition Coefficient - are relevant to gas chromatography (GC). The critical temperature of a gas (option D) is also relevant as it affects the chromatographic separation. Therefore, the correct answer is option 'D' - A, B, and C only.
Let's discuss each option in detail and understand why they are relevant to gas chromatography (GC):
A. Electron Capture:
Electron capture is a detection technique used in gas chromatography. In this technique, a radioactive source emits beta particles, which are captured by the analyte molecules in the gas phase. This leads to the formation of negative ions, which can be detected and measured. Electron capture is particularly useful for the detection of electron-capturing compounds such as halogenated compounds, nitro compounds, and some pesticides.
B. Deemter Equation:
The Deemter equation is an empirical equation that describes the relationship between plate height (H) and linear flow velocity (u) in a chromatographic column. The equation is given as H = A + Bu + Cu^2, where A, B, and C are constants. The Deemter equation is used to optimize the separation efficiency of a chromatographic column by balancing the contributions of different factors such as longitudinal diffusion, resistance to mass transfer, and eddy diffusion.
C. Partition Coefficient:
Partition coefficient, also known as distribution coefficient, is a measure of the distribution of a solute between two immiscible phases, such as a stationary phase and a mobile phase in gas chromatography. It is defined as the ratio of the concentration of the solute in the stationary phase to the concentration of the solute in the mobile phase at equilibrium. The partition coefficient determines the degree of retention of a solute in the stationary phase, which affects its separation and elution time in gas chromatography.
D. Critical Temperature of Gas:
The critical temperature of a gas is the temperature above which the gas cannot be liquefied, regardless of the pressure. It is an important property to consider in gas chromatography because it determines the operating conditions for the separation. The critical temperature of a gas influences its behavior in the chromatographic column and affects factors such as diffusion, mass transfer, and retention time.
In conclusion, all the options A, B, and C - Electron Capture, Deemter Equation, and Partition Coefficient - are relevant to gas chromatography (GC). The critical temperature of a gas (option D) is also relevant as it affects the chromatographic separation. Therefore, the correct answer is option 'D' - A, B, and C only.

|
Explore Courses for UGC NET exam
|

|
Question Description
Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer?.
Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Consider the following with regard to gas chromatography (GC).A. Electron captureB. Deemter equationC. Partition CoefficientD. Critical temperature of gasOf the above, which are relevant to GC?Choose the correct answer from the options given below:a)A, B and D onlyb)A, C and D onlyc)B, C and D onlyd)A, B and C onlyCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















