Class 6 Exam > Class 6 Questions > Most of the substances that we see around us ...
Start Learning for Free
Most of the substances that we see around us are:
- a)Element
- b)Compound
- c)Mixture
- d)Pure solution
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Most of the substances that we see around us are:a)Elementb)Compoundc)...
Understanding Substances Around Us
Most of the substances we encounter daily are classified as mixtures. Here’s why:
What is a Mixture?
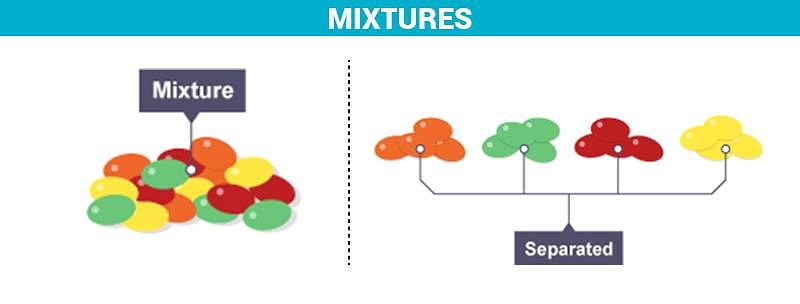
- A mixture is a combination of two or more substances that retain their individual properties.
- Examples include air, salad, and soil, where components can be easily separated.
Why are Mixtures Common?
- Natural Occurrence: Many substances found in nature are mixtures. For instance, river water contains various dissolved minerals, organic matter, and microorganisms.
- Everyday Products: Most household items like beverages, food, and cleaning supplies are mixtures. For example, a smoothie contains fruits, yogurt, and ice, each maintaining its properties.
Comparison with Other Substances
- Elements: These are pure substances that consist of only one type of atom, like oxygen or gold. While important, they are less common in everyday observations.
- Compounds: Compounds are formed when two or more elements chemically combine, like water (H₂O) or table salt (NaCl). They have fixed compositions and properties, making them less diverse in daily life.
- Pure Solutions: These are homogeneous mixtures where the solute is completely dissolved in the solvent, like sugar water. However, they are just one type of mixture and not as frequently encountered as heterogeneous mixtures.
Conclusion
In summary, while elements and compounds are essential in chemistry, the varied and complex nature of mixtures makes them the most common substances in our surroundings.
Most of the substances we encounter daily are classified as mixtures. Here’s why:
What is a Mixture?
- A mixture is a combination of two or more substances that retain their individual properties.
- Examples include air, salad, and soil, where components can be easily separated.
Why are Mixtures Common?
- Natural Occurrence: Many substances found in nature are mixtures. For instance, river water contains various dissolved minerals, organic matter, and microorganisms.
- Everyday Products: Most household items like beverages, food, and cleaning supplies are mixtures. For example, a smoothie contains fruits, yogurt, and ice, each maintaining its properties.
Comparison with Other Substances
- Elements: These are pure substances that consist of only one type of atom, like oxygen or gold. While important, they are less common in everyday observations.
- Compounds: Compounds are formed when two or more elements chemically combine, like water (H₂O) or table salt (NaCl). They have fixed compositions and properties, making them less diverse in daily life.
- Pure Solutions: These are homogeneous mixtures where the solute is completely dissolved in the solvent, like sugar water. However, they are just one type of mixture and not as frequently encountered as heterogeneous mixtures.
Conclusion
In summary, while elements and compounds are essential in chemistry, the varied and complex nature of mixtures makes them the most common substances in our surroundings.
Free Test
FREE
| Start Free Test |
Community Answer
Most of the substances that we see around us are:a)Elementb)Compoundc)...
Most of the substances that we see around us are a mixture of two or more substances.
Attention Class 6 Students!
To make sure you are not studying endlessly, EduRev has designed Class 6 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 6.

|
Explore Courses for Class 6 exam
|

|
Similar Class 6 Doubts
Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer?
Question Description
Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer? for Class 6 2024 is part of Class 6 preparation. The Question and answers have been prepared according to the Class 6 exam syllabus. Information about Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 6 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer?.
Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer? for Class 6 2024 is part of Class 6 preparation. The Question and answers have been prepared according to the Class 6 exam syllabus. Information about Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 6 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 6.
Download more important topics, notes, lectures and mock test series for Class 6 Exam by signing up for free.
Here you can find the meaning of Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Most of the substances that we see around us are:a)Elementb)Compoundc)Mixtured)Pure solutionCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 6 tests.

|
Explore Courses for Class 6 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























