UGC NET Exam > UGC NET Questions > The correct order of O-O bond length in O2,H2...
Start Learning for Free
The correct order of O-O bond length in O2, H2O2 and O3 is:
- a)O2>O3>H2O2
- b)H2O2>O3>O2
- c)O3>O2> H2O2
- d)O3>H2O2>O2
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>...
- The order of O-O bond length is generally dependent on the number of bonds between the oxygen atoms. A single bond is longer than a double bond, and a double bond is longer than a triple bond due to the increased electron density between the two atoms.
- Molecular Orbital Theory (MOT) provides a diagrammatic representation of the distribution of electrons in molecules. According to this theory, when atoms combine, the number of atomic orbitals remains the same but they transform into an equal number of molecular orbitals.
- The bond order in MOT is defined as half the difference between the number of bonding and antibonding electrons. Mathematically, it can be shown as:
- Bond Order = [Nb - Na] / 2 , where Nb denotes the number of electrons in the bonding molecular orbitals, and Na indicates the number of electrons in the antibonding molecular orbitals.
- O2: According to MO theory, O2 has a double bond, which is made up of one sigma and one pi bond. The presence of these stronger bonds leads to a shorter bond length.
- H2O2: The O-O bond in hydrogen peroxide is a single bond. So according to MO theory, it would be a sigma bond only, which results in a longer bond length compared with a double bond or a bond with resonance.
- O3: In ozone, due to the resonance of single and double bonds, the O-O bond length is somewhere between that of a single and double bond. So, the length of the bond in O3 is less than in H2O2 but greater than in O2.
- So, as per the molecular orbital theory as well, the order of O-O bond length, from longest to shortest should be:
H2O2 > O3 > O2
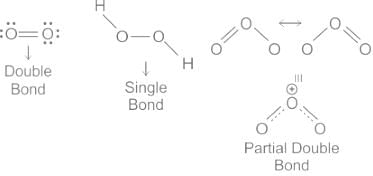
So, The correct order of O-O bond length in O2, H2O2 and O3 is H2O2 > O3 > O2.

So, The correct order of O-O bond length in O2, H2O2 and O3 is H2O2 > O3 > O2.
Most Upvoted Answer
The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>...
Explanation:
Oxygen Molecules (O2)
- O2 has a double bond between two oxygen atoms.
- The bond length in O2 is shorter compared to other oxygen-containing molecules due to the presence of a double bond.
Hydrogen Peroxide (H2O2)
- H2O2 has two O-O single bonds.
- The presence of single bonds in H2O2 results in longer bond lengths compared to O2.
Ozone (O3)
- O3 has a resonance structure with one double bond and one single bond.
- The presence of a single bond in O3 leads to longer bond lengths compared to O2 but shorter than H2O2.
Therefore, the correct order of O-O bond length is H2O2 > O3 > O2.
Oxygen Molecules (O2)
- O2 has a double bond between two oxygen atoms.
- The bond length in O2 is shorter compared to other oxygen-containing molecules due to the presence of a double bond.
Hydrogen Peroxide (H2O2)
- H2O2 has two O-O single bonds.
- The presence of single bonds in H2O2 results in longer bond lengths compared to O2.
Ozone (O3)
- O3 has a resonance structure with one double bond and one single bond.
- The presence of a single bond in O3 leads to longer bond lengths compared to O2 but shorter than H2O2.
Therefore, the correct order of O-O bond length is H2O2 > O3 > O2.

|
Explore Courses for UGC NET exam
|

|
Similar UGC NET Doubts
Question Description
The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer?.
The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer?.
Solutions for The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer?, a detailed solution for The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The correct order of O-O bond length in O2,H2O2and O3is:a)O2>O3>H2O2b)H2O2>O3>O2c)O3>O2> H2O2d)O3>H2O2>O2Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



























