UGC NET Exam > UGC NET Questions > Identify the wrong statement among the follow...
Start Learning for Free
Identify the wrong statement among the following
- a)All bonds between Ni and C in [Ni(CO)4] are not equivalent
- b)Metal carbonyl complexes are belongs to organometallic compounds
- c)Fe3(CO)12 contains three bridged carbonyls
- d)Mn does not form mononuclear carbonyls
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Identify the wrong statement among the following a)All bonds between N...
Metal atoms bonded to carbon monoxide (CO) form metal carbonyls. The first metal carbonyl prepared was nickel tetracarbonyl-Ni(CO)4, which was obtained by Mond in 1890. Since this complex contains only one metal atom and carbonyl ligands, they are also known as binary metal carbonyls.
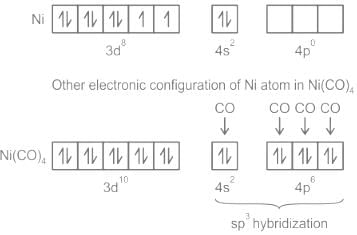
- In Ni(CO)4, the ligands (CO) are neutral molecules and the metals are present in the zero oxidation state. Nickel with an atomic number of 28 and zero oxidation state has 4s2 3d8 as the outer electronic configuration. As CO is a strong ligand, it shifts electrons from 4s to 3d and hence 4s + 4p orbitals undergo sp3 hybridisation to form tetrahedral geometry. Fig (a). formation of sp3 hybrid orbitals; (b) tetrahedral geometry of Ni(CO)4
(a)
(b)
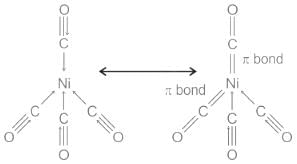
- The M-C bond in metal carbonyl has σ as well as π character. The metal-carbon σ bond is formed by the donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of Ni because of which the metal acquires a negative formal charge. In order to remove part of this negative charge, the electron density is donated back from the filled d-orbital of metal into the vacant antibonding π* orbitals of carbon monoxide. This results in a slight double bond character to the Ni-CO bond and a slight reduction from the triple bond character in the CO ligand.
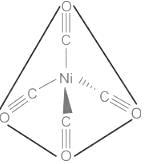
- From the structure of Nickel tetracarbonyl, it can be seen that two out of four CO groups are linked to Nickel through sigma bonds, and the remaining two are linked through multiple bonds (σ + π). Therefore, all bonds between Ni and C between Ni(CO)4 are not equivalent.
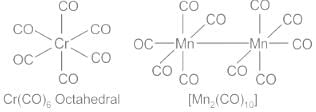
Carbon monoxide is one of the most important ligands in organometallic chemistry. Metal carbonyls are formed by reactions involving CO and either free metals or metal salts, and are characterised as having CO groups directly bonded to the metals through carbon. For example, [Cr(CO)6], [Mn2(CO)10]

- Most of the metal carbonyls have the metal in very low oxidation states, sometimes even zero.
- Organometallic compounds are compounds that should have at least one metal-carbon bond. Several transition metals or their compounds react with organic molecules forming organometallic compounds.
- Hence, metal carbonyls belong to organometallic compounds.
Metal carbonyls are broadly classified into two types, namely, mononuclear and polynuclear metal carbonyls.
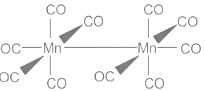
- Mononuclear metal carbonyls are those which contain only one central metal atom. Examples are [Ni(CO40], [V(CO)6}. Polynuclear metal carbonyls contain more than one central atom and further can be classified into binuclear, trinuclear, tetranuclear metal carbonyls and so on depending upon the number of central metal atoms. Examples: [Mn2(CO)10] - binuclear; [Fe3(CO)12] - trinuclear; [Co4(CO)12] - tetranuclear
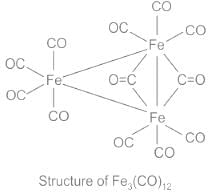
- The structural determination of Fe3(CO)12 has been quite challenging. After quite some spectral studies, the structure of this complex has been interpreted. In this structure, the three ion atoms are at the corners of an isosceles triangle. Ten of the CO ligands are terminal and two bridging CO groups are unsymmetrically placed.
- CO typically bonds in an end-on way through carbon. Yet bridging carbonyls are also found. Symmetrical and unsymmetrical bridging are observed within bridging carbonyls.
- Therefore, Fe3(CO)12 contains two bridged carbonyl
 types of bridging in metal carbonyls
types of bridging in metal carbonyls
Manganese belongs to the 3d series of the transition elements of the periodic table. Transition metals are known to form organometallic compounds and metal carbonyls are included in the organometallic compounds. Depending upon the number of central metal atoms, metal carbonyls are classified into mononuclear, binuclear, trinuclear and so on.
- Manganese with atomic number 28, has electronic configurations as:
1s2 2s2 2p6 3s2 3p6 3d5 4s2 and outer electronic configuration as 3d5 4s2 - CO ligand contributes 2e- from its lone pair. If we consider Mn(CO)5 fragment, the total contribution from the CO ligands is 5 × 2e- = 10e-. Mn has 7e- in the outermost shells, so the total electron count complex is 17e-. To complete the nearest noble gas configuration of Argon (18), the fragment dimerises to make the M-M bond because the unpaired electron in each fragment is shared with the other in forming the bond.
- Therefore, Mn2(CO)10 is the simplest metal-carbonyl satisfying the eighteen-electron rule and since this complex contains an Mn-Mn bond, it is a binuclear metal carbonyl. So Mn does not form mononuclear carbonyl.
Out of all the statements given above, Fe3(CO)12 contains three bridged carbonyls is the wrong statement as this complex contains only two bridged carbonyls as per spectral studies and interpretations.
Most Upvoted Answer
Identify the wrong statement among the following a)All bonds between N...
Fe3(CO)12 contains three bridged carbonyls
Metal carbonyl complexes typically have metal-carbon bonds with CO ligands, forming a class of organometallic compounds. However, the statement that Fe3(CO)12 contains three bridged carbonyls is incorrect.
Explanation:
- Fe3(CO)12 is a cluster compound of iron with 12 CO ligands.
- In Fe3(CO)12, there are no bridged carbonyls present.
- Each iron atom in Fe3(CO)12 is coordinated to four CO ligands, resulting in a total of 12 CO ligands in the compound.
- The CO ligands in Fe3(CO)12 are terminal, meaning they are directly bonded to the iron atoms and not bridging between them.
- Therefore, the statement that Fe3(CO)12 contains three bridged carbonyls is inaccurate.
In summary, Fe3(CO)12 does not have bridged carbonyls and instead consists of terminal CO ligands bonded to the iron atoms in the cluster compound.
Metal carbonyl complexes typically have metal-carbon bonds with CO ligands, forming a class of organometallic compounds. However, the statement that Fe3(CO)12 contains three bridged carbonyls is incorrect.
Explanation:
- Fe3(CO)12 is a cluster compound of iron with 12 CO ligands.
- In Fe3(CO)12, there are no bridged carbonyls present.
- Each iron atom in Fe3(CO)12 is coordinated to four CO ligands, resulting in a total of 12 CO ligands in the compound.
- The CO ligands in Fe3(CO)12 are terminal, meaning they are directly bonded to the iron atoms and not bridging between them.
- Therefore, the statement that Fe3(CO)12 contains three bridged carbonyls is inaccurate.
In summary, Fe3(CO)12 does not have bridged carbonyls and instead consists of terminal CO ligands bonded to the iron atoms in the cluster compound.

|
Explore Courses for UGC NET exam
|

|
Similar UGC NET Doubts
Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer?
Question Description
Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer?.
Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Identify the wrong statement among the following a)All bonds between Ni and C in [Ni(CO)4] are not equivalentb)Metal carbonyl complexes are belongs to organometallic compoundsc)Fe3(CO)12 contains three bridged carbonylsd)Mn does not form mononuclear carbonylsCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























