UGC NET Exam > UGC NET Questions > Consider the following statements-(A) Element...
Start Learning for Free
Consider the following statements-
(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.
(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.
(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.
The correct statement(s) are
(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.
(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.
(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.
The correct statement(s) are
- a)A only
- b)A and C only
- c)A and B only
- d)A, B, and C
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Consider the following statements-(A) Elemental sulfur forms rings or ...
- Catenation is defined as the ability of a chemical element to form a long chain-like structure via a series of covalent bonds. Oxygen’s extent of catenation is limited to ozone (O3) and peroxides (e.g., R-O-O-R). In contrast, the chemistry of sulfur is rich in the formation of multiple S-S bonds.
- While elemental sulfur exists as a diatomic molecule (i.e., S2) in the gas phase at high temperatures, sulfur vapor consists of a mixture of oligomers (S3 to S8) as a temperature dependant equilibrium. In the solid state, the formation of Sn dominates, and sulfur exists as a range of polymorphs in which extended S-S bonding occurs in either ring of 6 to 20 atoms or chains (catenasulfur).
- Thus, Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules. So statement A is correct.
- Phosphorous acid is the compound described by the formula H3PO3. This acid is dibasic acid.
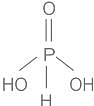
- Thus, statement B is incorrect.
- Diborane (B2H6) is an electron-deficient species. It contains less number of electrons than required for bonding. Whereas phosphine is an electron-rich compound.
- In diborane, 14 electrons are required to form 7 bonds. But in diborane, only 6 bonds are present due to insufficient electrons.
- Thus statement C is also correct.
Hence, The correct statement(s) are A and C only.

|
Explore Courses for UGC NET exam
|

|
Similar UGC NET Doubts
Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer?
Question Description
Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer?.
Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Consider the following statements-(A) Elemental sulfur forms rings or chains with S-S single bonds and oxygen exists as diatomic molecules.(B) Phosphorous acid is monoprotic acid and phosphoric acid is tribasic acid.(C) Phosphene is an electron-rich molecular compound whereas diborane is an electron-deficient compound.The correct statement(s) area)A onlyb)A and C onlyc)A and B onlyd)A, B, and CCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























