UGC NET Exam > UGC NET Questions > The catalyst and co-catalyst used in the Wack...
Start Learning for Free
The catalyst and co-catalyst used in the Wacker process, respectively, are.
- a)PdCl2 and Cu
- b)Pd and CuCl
- c)CuCl2 and [PdCl4]2-
- d)[PdCl4]2- and CuCl2
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
The catalyst and co-catalyst used in the Wacker process, respectively,...
[PdCl4]2- is used as a catalyst during the reaction which transforms to Pd (0). Co-catalyst CuCl2 is used to convert Pd (0) to Pd (II)
The initial stoichiometric reaction was first reported by Phillips.The net reaction can also be described as follows:
[PdCl4]2 − + C2H4 + H2O → CH3CHO + Pd + 2HCl + 2 Cl−
This conversion is followed by reactions that regenerate the Pd(II) catalyst:
Pd + 2 CuCl2 + 2 Cl − → [PdCl4]2− + 2 CuCl
2 CuCl + 1/2 O2 + 2 HCl → 2 CuCl2 + H2O
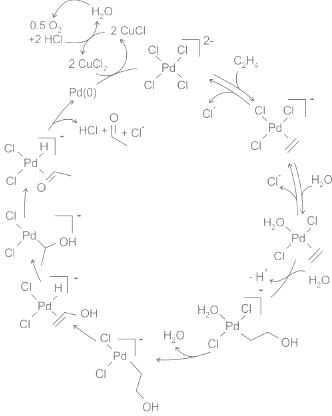
So, The catalyst and co-catalyst used in the Wacker process, respectively, are [PdCl4]2- and CuCl2.
The initial stoichiometric reaction was first reported by Phillips.The net reaction can also be described as follows:
[PdCl4]2 − + C2H4 + H2O → CH3CHO + Pd + 2HCl + 2 Cl−
This conversion is followed by reactions that regenerate the Pd(II) catalyst:
Pd + 2 CuCl2 + 2 Cl − → [PdCl4]2− + 2 CuCl
2 CuCl + 1/2 O2 + 2 HCl → 2 CuCl2 + H2O

So, The catalyst and co-catalyst used in the Wacker process, respectively, are [PdCl4]2- and CuCl2.
Most Upvoted Answer
The catalyst and co-catalyst used in the Wacker process, respectively,...
The Wacker Process and its Catalysts
The Wacker process is a chemical reaction used to convert ethylene into acetaldehyde. This reaction is industrially significant because acetaldehyde is a key intermediate in the production of various chemicals and materials, such as acetic acid, plastics, and pharmaceuticals.
The Wacker process involves the oxidation of ethylene using a catalyst system consisting of a precious metal catalyst and a co-catalyst. The catalyst and co-catalyst work together to facilitate the reaction and increase its efficiency.
The Catalyst: [PdCl4]2-
The catalyst used in the Wacker process is [PdCl4]2-, which is a palladium complex. Palladium is a transition metal that is known for its catalytic properties, particularly in oxidation reactions. In this case, the palladium catalyst facilitates the conversion of ethylene to acetaldehyde by promoting the transfer of oxygen atoms from the co-catalyst to the ethylene molecule.
The [PdCl4]2- complex is soluble in the reaction medium and can undergo reversible redox reactions, which are crucial for the catalytic cycle. The palladium catalyst can be regenerated by reoxidation, allowing it to participate in multiple reaction cycles, thus increasing the overall efficiency of the process.
The Co-catalyst: CuCl2
The co-catalyst used in the Wacker process is CuCl2, which is a copper salt. Copper is another transition metal that is commonly used in catalysis. In this case, the co-catalyst plays a crucial role in the reaction by providing the necessary oxygen atoms for the oxidation of ethylene.
CuCl2 acts as an oxygen carrier, helping to transfer oxygen atoms to the palladium catalyst. It undergoes reversible redox reactions, allowing it to participate in the catalytic cycle. The co-catalyst can be regenerated by reoxidation, enabling it to continue providing oxygen atoms for the oxidation of ethylene.
Synergistic Effect
The combination of the palladium catalyst and the copper co-catalyst in the Wacker process creates a synergistic effect. The palladium catalyst facilitates the conversion of ethylene to acetaldehyde, while the copper co-catalyst provides the necessary oxygen atoms for the reaction. This cooperative interaction between the catalyst and co-catalyst enhances the efficiency of the process and allows for the production of acetaldehyde on an industrial scale.
Overall, the use of [PdCl4]2- as the catalyst and CuCl2 as the co-catalyst in the Wacker process is a well-established and effective catalytic system. This combination harnesses the catalytic properties of both palladium and copper, allowing for the efficient conversion of ethylene into acetaldehyde.
The Wacker process is a chemical reaction used to convert ethylene into acetaldehyde. This reaction is industrially significant because acetaldehyde is a key intermediate in the production of various chemicals and materials, such as acetic acid, plastics, and pharmaceuticals.
The Wacker process involves the oxidation of ethylene using a catalyst system consisting of a precious metal catalyst and a co-catalyst. The catalyst and co-catalyst work together to facilitate the reaction and increase its efficiency.
The Catalyst: [PdCl4]2-
The catalyst used in the Wacker process is [PdCl4]2-, which is a palladium complex. Palladium is a transition metal that is known for its catalytic properties, particularly in oxidation reactions. In this case, the palladium catalyst facilitates the conversion of ethylene to acetaldehyde by promoting the transfer of oxygen atoms from the co-catalyst to the ethylene molecule.
The [PdCl4]2- complex is soluble in the reaction medium and can undergo reversible redox reactions, which are crucial for the catalytic cycle. The palladium catalyst can be regenerated by reoxidation, allowing it to participate in multiple reaction cycles, thus increasing the overall efficiency of the process.
The Co-catalyst: CuCl2
The co-catalyst used in the Wacker process is CuCl2, which is a copper salt. Copper is another transition metal that is commonly used in catalysis. In this case, the co-catalyst plays a crucial role in the reaction by providing the necessary oxygen atoms for the oxidation of ethylene.
CuCl2 acts as an oxygen carrier, helping to transfer oxygen atoms to the palladium catalyst. It undergoes reversible redox reactions, allowing it to participate in the catalytic cycle. The co-catalyst can be regenerated by reoxidation, enabling it to continue providing oxygen atoms for the oxidation of ethylene.
Synergistic Effect
The combination of the palladium catalyst and the copper co-catalyst in the Wacker process creates a synergistic effect. The palladium catalyst facilitates the conversion of ethylene to acetaldehyde, while the copper co-catalyst provides the necessary oxygen atoms for the reaction. This cooperative interaction between the catalyst and co-catalyst enhances the efficiency of the process and allows for the production of acetaldehyde on an industrial scale.
Overall, the use of [PdCl4]2- as the catalyst and CuCl2 as the co-catalyst in the Wacker process is a well-established and effective catalytic system. This combination harnesses the catalytic properties of both palladium and copper, allowing for the efficient conversion of ethylene into acetaldehyde.

|
Explore Courses for UGC NET exam
|

|
Question Description
The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer?.
The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer?.
Solutions for The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer?, a detailed solution for The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The catalyst and co-catalyst used in the Wacker process, respectively, are.a)PdCl2 and Cub)Pd and CuClc)CuCl2 and [PdCl4]2-d)[PdCl4]2- and CuCl2Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.




















