UGC NET Exam > UGC NET Questions > According to transition state theory, the tem...
Start Learning for Free
According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given by
- a)T
- b)T2
- c)T-2
- d)T-1.5
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
According to transition state theory, the temperature-dependence pf pr...
Explanation:
Transition state theory is a theory that describes the transition state that occurs during a chemical reaction. It provides a framework for understanding the kinetics of reactions and how they are affected by temperature.
The temperature-dependence of the pre-exponential factor (A) for a reaction can be determined using the Arrhenius equation, which relates the rate constant (k) of a reaction to the temperature (T) and the activation energy (Ea):
k = A * e^(-Ea/RT)
where R is the gas constant.
Temperature-dependence of the pre-exponential factor (A):
The pre-exponential factor (A) represents the frequency at which reactant molecules collide with enough energy to overcome the activation energy barrier and form products. It depends on the steric requirements of the reaction, the geometry of the transition state, and the number of collisions that result in successful reactions.
In the given question, the reaction occurs between a linear and non-linear molecule, and the product is formed through a non-linear transition state. This implies that the reaction involves a change in molecular geometry during the transition state.
Effect of temperature on the pre-exponential factor:
The pre-exponential factor (A) is influenced by the rate of collision between reactant molecules. As temperature increases, the kinetic energy of the molecules also increases, leading to an increase in the frequency of collisions.
In the case of a reaction involving a non-linear transition state, the steric requirements and geometry of the transition state are more complex compared to a linear transition state. This complexity leads to a decrease in the number of successful collisions and therefore a decrease in the pre-exponential factor (A) with increasing temperature.
Temperature dependence of A:
The temperature-dependence of the pre-exponential factor (A) for a reaction between a linear and non-linear molecule, forming a product through a non-linear transition state, is given by T^(-1.5). This means that as temperature increases, the pre-exponential factor decreases with the square root of the temperature.
Therefore, the correct answer is option 'D' - T^(-1.5).
Transition state theory is a theory that describes the transition state that occurs during a chemical reaction. It provides a framework for understanding the kinetics of reactions and how they are affected by temperature.
The temperature-dependence of the pre-exponential factor (A) for a reaction can be determined using the Arrhenius equation, which relates the rate constant (k) of a reaction to the temperature (T) and the activation energy (Ea):
k = A * e^(-Ea/RT)
where R is the gas constant.
Temperature-dependence of the pre-exponential factor (A):
The pre-exponential factor (A) represents the frequency at which reactant molecules collide with enough energy to overcome the activation energy barrier and form products. It depends on the steric requirements of the reaction, the geometry of the transition state, and the number of collisions that result in successful reactions.
In the given question, the reaction occurs between a linear and non-linear molecule, and the product is formed through a non-linear transition state. This implies that the reaction involves a change in molecular geometry during the transition state.
Effect of temperature on the pre-exponential factor:
The pre-exponential factor (A) is influenced by the rate of collision between reactant molecules. As temperature increases, the kinetic energy of the molecules also increases, leading to an increase in the frequency of collisions.
In the case of a reaction involving a non-linear transition state, the steric requirements and geometry of the transition state are more complex compared to a linear transition state. This complexity leads to a decrease in the number of successful collisions and therefore a decrease in the pre-exponential factor (A) with increasing temperature.
Temperature dependence of A:
The temperature-dependence of the pre-exponential factor (A) for a reaction between a linear and non-linear molecule, forming a product through a non-linear transition state, is given by T^(-1.5). This means that as temperature increases, the pre-exponential factor decreases with the square root of the temperature.
Therefore, the correct answer is option 'D' - T^(-1.5).
Free Test
FREE
| Start Free Test |
Community Answer
According to transition state theory, the temperature-dependence pf pr...
Transition State Theory; Transition state theory is a fundamental concept in chemical kinetics that describes the formation of an activated complex or transition state in a chemical reaction. It provides insights into reaction mechanisms, activation energies, and rate constants.
Arrhenius Equation: The Arrhenius equation is commonly used in chemical kinetics to relate the rate constant (k) to temperature (T), activation energy (Ea), and the pre-exponential factor (A). It is a powerful tool for understanding how reaction rates depend on temperature.
Temperature Dependence of A : In the Arrhenius equation, the pre-exponential factor (A) represents the rate constant at a specific temperature. The temperature dependence of A is typically exponential, but in this case, it is specified as T(-1.5), meaning that A decreases as temperature increases to the power of -1.5.
Unusual Temperature Dependence: The temperature dependence of A is usually expressed as an exponential function, with A increasing as temperature rises. However, in the given scenario involving a linear and non-linear molecule forming a product through a non-linear transition state, the temperature dependence of A is specified as T(-1.5), which represents an unusual and non-standard temperature dependence. This suggests a unique kinetic behavior for such reactions.
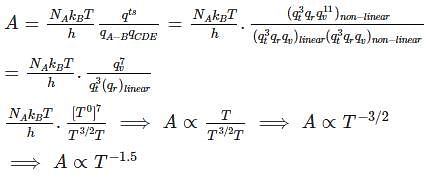
So, The correct answer is T-1.5.
Arrhenius Equation: The Arrhenius equation is commonly used in chemical kinetics to relate the rate constant (k) to temperature (T), activation energy (Ea), and the pre-exponential factor (A). It is a powerful tool for understanding how reaction rates depend on temperature.
Temperature Dependence of A : In the Arrhenius equation, the pre-exponential factor (A) represents the rate constant at a specific temperature. The temperature dependence of A is typically exponential, but in this case, it is specified as T(-1.5), meaning that A decreases as temperature increases to the power of -1.5.
Unusual Temperature Dependence: The temperature dependence of A is usually expressed as an exponential function, with A increasing as temperature rises. However, in the given scenario involving a linear and non-linear molecule forming a product through a non-linear transition state, the temperature dependence of A is specified as T(-1.5), which represents an unusual and non-standard temperature dependence. This suggests a unique kinetic behavior for such reactions.
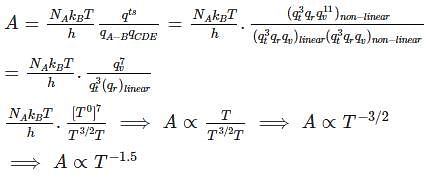
So, The correct answer is T-1.5.
Attention UGC NET Students!
To make sure you are not studying endlessly, EduRev has designed UGC NET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in UGC NET.

|
Explore Courses for UGC NET exam
|

|
Similar UGC NET Doubts
According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer?
Question Description
According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer? for UGC NET 2024 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for UGC NET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer?.
According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer? for UGC NET 2024 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for UGC NET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer?.
Solutions for According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer?, a detailed solution for According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice According to transition state theory, the temperature-dependence pf pre-exponential factor(A) for a reaction between a linear and non linear molecu;e, tat forms a product through a non-linear transition state, is given bya)Tb)T2c)T-2d)T-1.5Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























