UGC NET Exam > UGC NET Questions > Higher Michalis-Menten constant value of the ...
Start Learning for Free
Higher Michalis-Menten constant value of the enzyme often denotes
- a)Higher affinity of the enzyme to the substrate
- b)Lower affinity of the enzyme to the substrate
- c)Affinity is constant of the enzyme to the substrate analogue
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Higher Michalis-Menten constant value of the enzyme often denotesa)Hig...
The Michaelis-Menten constant, often denoted by Km, is a fundamental concept in enzyme kinetics. It quantitatively analyzes how enzymes act as biological catalysts to speed up the rate of biochemical reactions.
The Km is the substrate concentration at which an enzyme reaches half of its maximum rate of reaction, or Vmax/2. It is determined experimentally from measurements of enzyme activity at various substrate concentrations and using a combination of the Michaelis-Menten equation and the Lineweaver-Burk plot.
Mathematically, Km values can be defined in terms of the rate constants of the enzyme kinetics. If k1 is the rate constant for the reaction of enzyme E and substrate S to form the enzyme-substrate complex ES, and k-1 is the rate constant for the reverse reaction (dissociation of ES back to E + S), then
Km = (k-1 + k2) / k1
where k2 is the rate constant for the conversion of ES to E and product P.
Importantly, Km provides information about the affinity of an enzyme for its substrate; a lower Km value indicates higher affinity because less substrate is needed for the enzyme to reach half of its maximum rate of reaction.
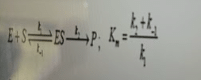
if Km is higher then K1 will lower
So the rate of complex formation is very low i.e enzyme has lower affinity to the substrate.
The Km is the substrate concentration at which an enzyme reaches half of its maximum rate of reaction, or Vmax/2. It is determined experimentally from measurements of enzyme activity at various substrate concentrations and using a combination of the Michaelis-Menten equation and the Lineweaver-Burk plot.
Mathematically, Km values can be defined in terms of the rate constants of the enzyme kinetics. If k1 is the rate constant for the reaction of enzyme E and substrate S to form the enzyme-substrate complex ES, and k-1 is the rate constant for the reverse reaction (dissociation of ES back to E + S), then
Km = (k-1 + k2) / k1
where k2 is the rate constant for the conversion of ES to E and product P.
Importantly, Km provides information about the affinity of an enzyme for its substrate; a lower Km value indicates higher affinity because less substrate is needed for the enzyme to reach half of its maximum rate of reaction.
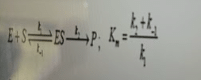
if Km is higher then K1 will lower
So the rate of complex formation is very low i.e enzyme has lower affinity to the substrate.
Most Upvoted Answer
Higher Michalis-Menten constant value of the enzyme often denotesa)Hig...
Explanation:
Michalis-Menten Constant:
The Michaelis-Menten constant (Km) is a measure of the enzyme's affinity for its substrate. It represents the substrate concentration at which an enzyme achieves half of its maximum reaction rate.
Higher Km Value:
- A higher Km value indicates lower affinity of the enzyme to the substrate. This means that the enzyme requires a higher concentration of substrate to reach half of its maximum reaction rate.
- Enzymes with higher Km values are considered to have a weaker binding affinity for the substrate.
Lower Affinity to Substrate:
- Therefore, in the context of enzyme kinetics, a higher Km value signifies lower affinity of the enzyme to the substrate.
- On the other hand, a lower Km value indicates higher affinity of the enzyme to the substrate, as the enzyme reaches half of its maximum reaction rate at lower substrate concentrations.
Conclusion:
- In summary, a higher Michalis-Menten constant value of an enzyme denotes lower affinity of the enzyme to the substrate. This understanding is crucial for studying enzyme kinetics and the interactions between enzymes and substrates.

|
Explore Courses for UGC NET exam
|

|
Question Description
Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer?.
Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Higher Michalis-Menten constant value of the enzyme often denotesa)Higher affinity of the enzyme to the substrateb)Lower affinity of the enzyme to the substratec)Affinity is constant of the enzyme to the substrate analogued)None of theseCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















