Class 11 Exam > Class 11 Questions > Can you explain the answer of this question b...
Start Learning for Free
Can you explain the answer of this question below:
The functional group given below is characteristic of organic _____ .
- A:ketones
- B:acids
- C:aldehydes
- D:esters
The answer is a.
Verified Answer
Can you explain the answer of this question below:The functional group...
Ketones
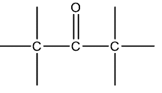
The functional group, which is present in a ketone is >C=O. The IUPAC group suffix of a ketone is –one.
Example: Acetone
 This question is part of UPSC exam. View all Class 11 courses
This question is part of UPSC exam. View all Class 11 courses
Most Upvoted Answer
Can you explain the answer of this question below:The functional group...
First post ur following functional group then I will tell u correct answer
Community Answer
Can you explain the answer of this question below:The functional group...
Functional Group: Ketones
Introduction:
The given question asks about the functional group that is characteristic of organic compounds. Among the options provided, the correct answer is ketones. In this response, we will discuss the characteristics of ketones and why they are the appropriate choice for the given functional group.
Characteristics of Ketones:
Ketones are organic compounds that contain a carbonyl group (C=O) bonded to two carbon atoms. They are characterized by the presence of the carbonyl functional group, which is responsible for their unique properties and reactivity. Some key characteristics of ketones include:
1. Carbonyl Group: The carbonyl group consists of a carbon atom double-bonded to an oxygen atom. In ketones, this carbonyl group is located within the carbon chain, with two alkyl or aryl groups attached to the carbonyl carbon.
2. Naming: Ketones are named by replacing the -e ending of the corresponding alkane with -one. For example, the ketone derived from propane is called propanone (commonly known as acetone).
3. Polarity: Ketones have a polar carbonyl group, which makes them relatively polar compounds overall. This polarity influences their physical and chemical properties, such as solubility in polar solvents like water.
4. Boiling Points: Ketones generally have higher boiling points compared to alkanes of similar molecular weight. This is due to the presence of the polar carbonyl group, which allows for dipole-dipole interactions between ketone molecules.
5. Reactivity: Ketones can undergo various chemical reactions due to the presence of the carbonyl group. These reactions include nucleophilic addition, oxidation, reduction, and condensation reactions.
Justification:
Based on the characteristics discussed above, it is clear that the functional group given in the question, the carbonyl group (C=O), is characteristic of ketones. This rules out options B (acids), C (aldehydes), and D (esters) as incorrect choices. Acids have a carboxyl functional group (-COOH), aldehydes have a carbonyl group at the end of the carbon chain, and esters have a carbonyl group bonded to an oxygen atom and another carbon atom. Therefore, the correct answer is option A: ketones.
Conclusion:
In conclusion, the functional group given in the question is characteristic of organic ketones. Ketones are organic compounds that contain a carbonyl group (C=O) bonded to two carbon atoms. They possess several unique characteristics, such as the polar carbonyl group, higher boiling points compared to alkanes, and reactivity towards various chemical reactions.
Introduction:
The given question asks about the functional group that is characteristic of organic compounds. Among the options provided, the correct answer is ketones. In this response, we will discuss the characteristics of ketones and why they are the appropriate choice for the given functional group.
Characteristics of Ketones:
Ketones are organic compounds that contain a carbonyl group (C=O) bonded to two carbon atoms. They are characterized by the presence of the carbonyl functional group, which is responsible for their unique properties and reactivity. Some key characteristics of ketones include:
1. Carbonyl Group: The carbonyl group consists of a carbon atom double-bonded to an oxygen atom. In ketones, this carbonyl group is located within the carbon chain, with two alkyl or aryl groups attached to the carbonyl carbon.
2. Naming: Ketones are named by replacing the -e ending of the corresponding alkane with -one. For example, the ketone derived from propane is called propanone (commonly known as acetone).
3. Polarity: Ketones have a polar carbonyl group, which makes them relatively polar compounds overall. This polarity influences their physical and chemical properties, such as solubility in polar solvents like water.
4. Boiling Points: Ketones generally have higher boiling points compared to alkanes of similar molecular weight. This is due to the presence of the polar carbonyl group, which allows for dipole-dipole interactions between ketone molecules.
5. Reactivity: Ketones can undergo various chemical reactions due to the presence of the carbonyl group. These reactions include nucleophilic addition, oxidation, reduction, and condensation reactions.
Justification:
Based on the characteristics discussed above, it is clear that the functional group given in the question, the carbonyl group (C=O), is characteristic of ketones. This rules out options B (acids), C (aldehydes), and D (esters) as incorrect choices. Acids have a carboxyl functional group (-COOH), aldehydes have a carbonyl group at the end of the carbon chain, and esters have a carbonyl group bonded to an oxygen atom and another carbon atom. Therefore, the correct answer is option A: ketones.
Conclusion:
In conclusion, the functional group given in the question is characteristic of organic ketones. Ketones are organic compounds that contain a carbonyl group (C=O) bonded to two carbon atoms. They possess several unique characteristics, such as the polar carbonyl group, higher boiling points compared to alkanes, and reactivity towards various chemical reactions.

|
Explore Courses for Class 11 exam
|

|
Question Description
Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a. for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a. covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a..
Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a. for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a. covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a..
Solutions for Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a. in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a. defined & explained in the simplest way possible. Besides giving the explanation of
Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a., a detailed solution for Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a. has been provided alongside types of Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a. theory, EduRev gives you an
ample number of questions to practice Can you explain the answer of this question below:The functional group given below is characteristic of organic _____ .A:ketonesB:acidsC:aldehydesD:estersThe answer is a. tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.




















