Chemistry Exam > Chemistry Questions > For epoxidation of alkene which will be pref...
Start Learning for Free
For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH?
Most Upvoted Answer
For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaO...
The Epoxidation of Alkenes: H2O2/ACOH vs H2O2/NaOH
Introduction
The epoxidation of alkenes is an important reaction in organic chemistry that involves the addition of an oxygen atom to a carbon-carbon double bond, forming an epoxide. This reaction is widely used in the synthesis of various organic compounds, including pharmaceuticals, agrochemicals, and fine chemicals. Two commonly used reagent systems for epoxidation are H2O2/ACOH (hydrogen peroxide/acetic acid) and H2O2/NaOH (hydrogen peroxide/sodium hydroxide). In this guide, we will discuss the differences between these two reagent systems and explain their preferences in epoxidation reactions.
H2O2/ACOH
- The H2O2/ACOH reagent system consists of hydrogen peroxide and acetic acid.
- Acetic acid acts as both a solvent and a catalyst in the reaction.
- The reaction proceeds via a peracid intermediate, which is formed by the reaction of hydrogen peroxide with acetic acid.
- The peracid intermediate then reacts with the alkene to form the desired epoxide product.
- This reagent system is commonly used for the epoxidation of a wide range of alkenes.
- It is particularly suitable for the epoxidation of electron-rich alkenes, such as those containing electron-donating substituents.
- The reaction conditions can be easily adjusted by varying the concentration of acetic acid, allowing control over the reaction rate and selectivity.
- However, the use of acetic acid as a catalyst can sometimes lead to side reactions, such as the formation of esters or acetals.
H2O2/NaOH
- The H2O2/NaOH reagent system consists of hydrogen peroxide and sodium hydroxide.
- Sodium hydroxide acts as a base in the reaction, facilitating the formation of the epoxide.
- The reaction proceeds via a concerted mechanism, where the alkene reacts directly with the peroxide ion formed from the reaction of hydrogen peroxide with sodium hydroxide.
- This reagent system is commonly used for the epoxidation of electron-deficient alkenes, such as those containing electron-withdrawing substituents.
- It is particularly suitable for the epoxidation of α,β-unsaturated carbonyl compounds, as it can proceed under mild reaction conditions.
- The use of sodium hydroxide as a base can also lead to side reactions, such as the formation of enols or other Michael addition products.
Conclusion
In conclusion, the choice between H2O2/ACOH and H2O2/NaOH for the epoxidation of alkenes depends on several factors, including the nature of the alkene substrate and the desired reaction conditions. H2O2/ACOH is generally preferred for the epoxidation of electron-rich alkenes, while H2O2/NaOH is more suitable for electron-deficient alkenes. However, it is important to consider the potential side reactions associated with each reagent system and to optimize the reaction conditions accordingly.
Introduction
The epoxidation of alkenes is an important reaction in organic chemistry that involves the addition of an oxygen atom to a carbon-carbon double bond, forming an epoxide. This reaction is widely used in the synthesis of various organic compounds, including pharmaceuticals, agrochemicals, and fine chemicals. Two commonly used reagent systems for epoxidation are H2O2/ACOH (hydrogen peroxide/acetic acid) and H2O2/NaOH (hydrogen peroxide/sodium hydroxide). In this guide, we will discuss the differences between these two reagent systems and explain their preferences in epoxidation reactions.
H2O2/ACOH
- The H2O2/ACOH reagent system consists of hydrogen peroxide and acetic acid.
- Acetic acid acts as both a solvent and a catalyst in the reaction.
- The reaction proceeds via a peracid intermediate, which is formed by the reaction of hydrogen peroxide with acetic acid.
- The peracid intermediate then reacts with the alkene to form the desired epoxide product.
- This reagent system is commonly used for the epoxidation of a wide range of alkenes.
- It is particularly suitable for the epoxidation of electron-rich alkenes, such as those containing electron-donating substituents.
- The reaction conditions can be easily adjusted by varying the concentration of acetic acid, allowing control over the reaction rate and selectivity.
- However, the use of acetic acid as a catalyst can sometimes lead to side reactions, such as the formation of esters or acetals.
H2O2/NaOH
- The H2O2/NaOH reagent system consists of hydrogen peroxide and sodium hydroxide.
- Sodium hydroxide acts as a base in the reaction, facilitating the formation of the epoxide.
- The reaction proceeds via a concerted mechanism, where the alkene reacts directly with the peroxide ion formed from the reaction of hydrogen peroxide with sodium hydroxide.
- This reagent system is commonly used for the epoxidation of electron-deficient alkenes, such as those containing electron-withdrawing substituents.
- It is particularly suitable for the epoxidation of α,β-unsaturated carbonyl compounds, as it can proceed under mild reaction conditions.
- The use of sodium hydroxide as a base can also lead to side reactions, such as the formation of enols or other Michael addition products.
Conclusion
In conclusion, the choice between H2O2/ACOH and H2O2/NaOH for the epoxidation of alkenes depends on several factors, including the nature of the alkene substrate and the desired reaction conditions. H2O2/ACOH is generally preferred for the epoxidation of electron-rich alkenes, while H2O2/NaOH is more suitable for electron-deficient alkenes. However, it is important to consider the potential side reactions associated with each reagent system and to optimize the reaction conditions accordingly.
Community Answer
For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaO...
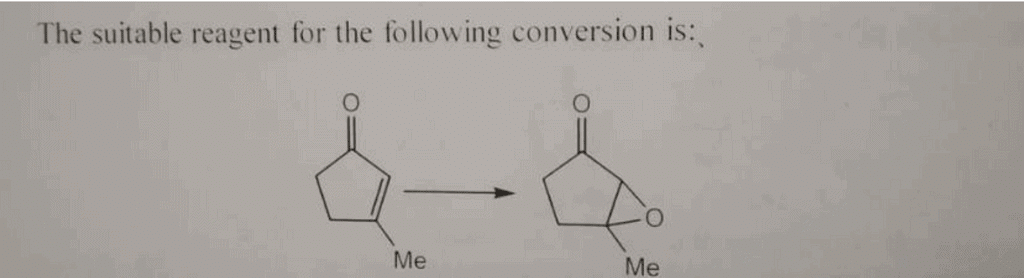

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Question Description
For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH?.
For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH?.
Solutions for For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH? defined & explained in the simplest way possible. Besides giving the explanation of
For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH?, a detailed solution for For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH? has been provided alongside types of For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH? theory, EduRev gives you an
ample number of questions to practice For epoxidation of alkene which will be prefer H2O2 /ACOH or H2O2/NaOH? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















