Software Development Exam > Software Development Questions > Which one of the following non-covalent inter...
Start Learning for Free
Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?
- a)A and B are permanent dipoles and are involved in hydrogen bonding.
- b)A and B are fully ionized and are involved in salt bridge formation.
- c)A and B are uncharged and repel each other.
- d)A and B are uncharged and attract each other.
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Which one of the following non-covalent interactions between two non-b...
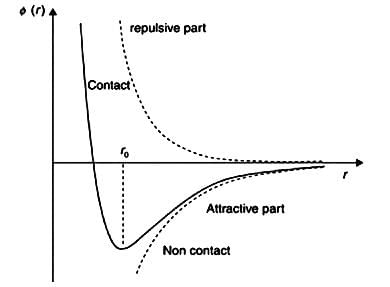
Van der Wall Interactions, also known as London forces are the weakest noncovalent interactions that can occur between two non-bonded atoms A and B. In absence of any other force, A and B atoms, while approaching each other tend to repel rather than attract one another. The distance is called van der wall contact distance. These are found to be very sensitive towards the distance. The attraction between two uncharged atoms A and B are inversely proportional to r6 (r= radius of atom), whereas repulsion between two atoms is more sensitive to distance as it is inversely proportional to r12. The repulsion takes place due to both atoms’ electron clouds hindering them from approaching each other closely.

Most Upvoted Answer
Which one of the following non-covalent interactions between two non-b...
Understanding Non-Covalent Interactions
Non-covalent interactions play a critical role in molecular interactions, particularly in biological systems. The sensitivity of these interactions to distance varies depending on the type of forces involved.
Types of Interactions
- **Permanent Dipoles and Hydrogen Bonding**: In this case, the interaction is strong but relies on the specific orientation and distance. However, hydrogen bonds have a relatively moderate distance sensitivity.
- **Salt Bridges**: Fully ionized atoms (like charged residues in proteins) exhibit strong electrostatic interactions. This interaction is sensitive to distance but also to the dielectric environment, making it less sensitive than other interactions at short distances due to screening effects.
- **Uncharged Repulsive Interactions**: When two uncharged atoms are close, they experience repulsion due to electron cloud overlap. This repulsion increases sharply as distance decreases, making it very sensitive to changes in distance.
- **Uncharged Attractive Interactions**: These interactions, like London dispersion forces, become significant at short distances but decrease rapidly with increasing separation.
Why Option C is the Most Sensitive
The interaction between uncharged atoms that repel each other (Option C) is exceedingly sensitive to distance because:
- **Rapid Increase in Repulsion**: As the distance decreases, the overlap of electron clouds escalates, leading to a sharp increase in repulsive forces.
- **Short-range Interaction**: Repulsive forces are strong at very short ranges, meaning even slight changes in distance can lead to substantial changes in energy.
- **Greater Impact on Stability**: The stability of non-covalent complexes is significantly affected by these repulsive interactions, making them critical to molecular behavior.
In summary, the repulsive forces between non-bonded, uncharged atoms (Option C) are the most sensitive to distance changes, demonstrating a pronounced impact on molecular interactions.
Non-covalent interactions play a critical role in molecular interactions, particularly in biological systems. The sensitivity of these interactions to distance varies depending on the type of forces involved.
Types of Interactions
- **Permanent Dipoles and Hydrogen Bonding**: In this case, the interaction is strong but relies on the specific orientation and distance. However, hydrogen bonds have a relatively moderate distance sensitivity.
- **Salt Bridges**: Fully ionized atoms (like charged residues in proteins) exhibit strong electrostatic interactions. This interaction is sensitive to distance but also to the dielectric environment, making it less sensitive than other interactions at short distances due to screening effects.
- **Uncharged Repulsive Interactions**: When two uncharged atoms are close, they experience repulsion due to electron cloud overlap. This repulsion increases sharply as distance decreases, making it very sensitive to changes in distance.
- **Uncharged Attractive Interactions**: These interactions, like London dispersion forces, become significant at short distances but decrease rapidly with increasing separation.
Why Option C is the Most Sensitive
The interaction between uncharged atoms that repel each other (Option C) is exceedingly sensitive to distance because:
- **Rapid Increase in Repulsion**: As the distance decreases, the overlap of electron clouds escalates, leading to a sharp increase in repulsive forces.
- **Short-range Interaction**: Repulsive forces are strong at very short ranges, meaning even slight changes in distance can lead to substantial changes in energy.
- **Greater Impact on Stability**: The stability of non-covalent complexes is significantly affected by these repulsive interactions, making them critical to molecular behavior.
In summary, the repulsive forces between non-bonded, uncharged atoms (Option C) are the most sensitive to distance changes, demonstrating a pronounced impact on molecular interactions.

|
Explore Courses for Software Development exam
|

|
Similar Software Development Doubts
Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer?
Question Description
Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer? for Software Development 2025 is part of Software Development preparation. The Question and answers have been prepared according to the Software Development exam syllabus. Information about Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Software Development 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer?.
Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer? for Software Development 2025 is part of Software Development preparation. The Question and answers have been prepared according to the Software Development exam syllabus. Information about Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Software Development 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer?.
Solutions for Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Software Development.
Download more important topics, notes, lectures and mock test series for Software Development Exam by signing up for free.
Here you can find the meaning of Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which one of the following non-covalent interactions between two non-bonded atoms A and B is most sensitive to the distance between them?a)A and B are permanent dipoles and are involved in hydrogen bonding.b)A and B are fully ionized and are involved in salt bridge formation.c)A and B are uncharged and repel each other.d)A and B are uncharged and attract each other.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Software Development tests.

|
Explore Courses for Software Development exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























