Class 12 Exam > Class 12 Questions > How to convert the reaction benzaldehyde to b...
Start Learning for Free
How to convert the reaction benzaldehyde to benzoic acid explain clearly?
Most Upvoted Answer
How to convert the reaction benzaldehyde to benzoic acid explain clear...
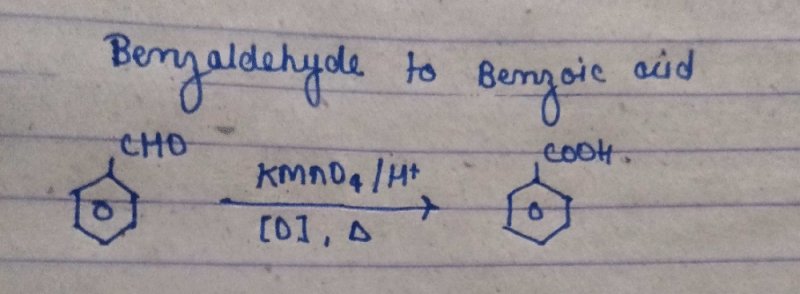
Community Answer
How to convert the reaction benzaldehyde to benzoic acid explain clear...
Conversion of Benzaldehyde to Benzoic Acid
To convert benzaldehyde to benzoic acid, a two-step oxidation reaction is required. Benzaldehyde, an aromatic aldehyde, undergoes oxidation to form benzoic acid, a carboxylic acid. The reaction involves the conversion of the aldehyde functional group to a carboxylic acid functional group.
Step 1: Oxidation of Benzaldehyde to Benzoic Acid using Tollens' Reagent
1. Prepare Tollens' reagent by dissolving silver nitrate (AgNO3) in distilled water, followed by adding a few drops of sodium hydroxide (NaOH) solution. The solution turns alkaline.
2. Take benzaldehyde in a clean test tube or flask.
3. Add Tollens' reagent dropwise to the benzaldehyde while continuously shaking or stirring the mixture.
4. Observe the reaction mixture. Initially, a silver mirror will form on the inner surface of the container. This is due to the reduction of silver ions (Ag+) to metallic silver (Ag) by benzaldehyde.
5. Continue adding Tollens' reagent until the silver mirror disappears completely.
6. The reaction is complete when the silver mirror disappears, indicating the conversion of benzaldehyde to benzoic acid.
Step 2: Acidification to Convert the Silver Mirror to Benzoic Acid
1. After the silver mirror disappears, add dilute hydrochloric acid (HCl) dropwise to the reaction mixture.
2. The silver mirror will dissolve in the presence of hydrochloric acid, forming a clear solution.
3. Heat the solution gently to evaporate excess water and concentrate the benzoic acid.
4. Allow the solution to cool and crystallize. Crystals of benzoic acid will form as the solution cools down.
5. Collect the benzoic acid crystals by filtration using a Buchner funnel or filter paper.
6. Wash the crystals with a small amount of cold water to remove any impurities.
7. Dry the benzoic acid crystals by placing them in an oven or desiccator to remove any remaining moisture.
Summary
By following these two oxidation steps, benzaldehyde can be converted to benzoic acid. The first step involves the oxidation of benzaldehyde using Tollens' reagent to form a silver mirror, which is then acidified to convert it into benzoic acid. The final product is obtained as crystalline benzoic acid after filtration and drying.
To convert benzaldehyde to benzoic acid, a two-step oxidation reaction is required. Benzaldehyde, an aromatic aldehyde, undergoes oxidation to form benzoic acid, a carboxylic acid. The reaction involves the conversion of the aldehyde functional group to a carboxylic acid functional group.
Step 1: Oxidation of Benzaldehyde to Benzoic Acid using Tollens' Reagent
1. Prepare Tollens' reagent by dissolving silver nitrate (AgNO3) in distilled water, followed by adding a few drops of sodium hydroxide (NaOH) solution. The solution turns alkaline.
2. Take benzaldehyde in a clean test tube or flask.
3. Add Tollens' reagent dropwise to the benzaldehyde while continuously shaking or stirring the mixture.
4. Observe the reaction mixture. Initially, a silver mirror will form on the inner surface of the container. This is due to the reduction of silver ions (Ag+) to metallic silver (Ag) by benzaldehyde.
5. Continue adding Tollens' reagent until the silver mirror disappears completely.
6. The reaction is complete when the silver mirror disappears, indicating the conversion of benzaldehyde to benzoic acid.
Step 2: Acidification to Convert the Silver Mirror to Benzoic Acid
1. After the silver mirror disappears, add dilute hydrochloric acid (HCl) dropwise to the reaction mixture.
2. The silver mirror will dissolve in the presence of hydrochloric acid, forming a clear solution.
3. Heat the solution gently to evaporate excess water and concentrate the benzoic acid.
4. Allow the solution to cool and crystallize. Crystals of benzoic acid will form as the solution cools down.
5. Collect the benzoic acid crystals by filtration using a Buchner funnel or filter paper.
6. Wash the crystals with a small amount of cold water to remove any impurities.
7. Dry the benzoic acid crystals by placing them in an oven or desiccator to remove any remaining moisture.
Summary
By following these two oxidation steps, benzaldehyde can be converted to benzoic acid. The first step involves the oxidation of benzaldehyde using Tollens' reagent to form a silver mirror, which is then acidified to convert it into benzoic acid. The final product is obtained as crystalline benzoic acid after filtration and drying.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
How to convert the reaction benzaldehyde to benzoic acid explain clearly?
Question Description
How to convert the reaction benzaldehyde to benzoic acid explain clearly? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about How to convert the reaction benzaldehyde to benzoic acid explain clearly? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How to convert the reaction benzaldehyde to benzoic acid explain clearly?.
How to convert the reaction benzaldehyde to benzoic acid explain clearly? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about How to convert the reaction benzaldehyde to benzoic acid explain clearly? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How to convert the reaction benzaldehyde to benzoic acid explain clearly?.
Solutions for How to convert the reaction benzaldehyde to benzoic acid explain clearly? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of How to convert the reaction benzaldehyde to benzoic acid explain clearly? defined & explained in the simplest way possible. Besides giving the explanation of
How to convert the reaction benzaldehyde to benzoic acid explain clearly?, a detailed solution for How to convert the reaction benzaldehyde to benzoic acid explain clearly? has been provided alongside types of How to convert the reaction benzaldehyde to benzoic acid explain clearly? theory, EduRev gives you an
ample number of questions to practice How to convert the reaction benzaldehyde to benzoic acid explain clearly? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















