JEE Exam > JEE Questions > Given below are two statements :Statement I: ...
Start Learning for Free
Given below are two statements :
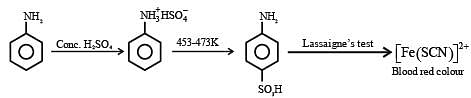
Statement I: Aniline reacts with con. H2SO4 followed by heating at 453-473 K gives P aminobenzene sulphonic acid, which gives blood red colour in the 'Lassaigne's test'.
Statement II: In Friedel - Craft's alkylation and acylation reactions, aniline forms salt with the AlCL3 catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.
In the light of the above statements, choose the correct answer from the options given below :
- a)Statement I is false but statement II is true
- b)Both statement I and statement II are false
- c)Statement I is true but statement II is false
- d)Both statement I and statement II are true
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Given below are two statements :Statement I: Aniline reacts with con. ...
Free Test
FREE
| Start Free Test |
Community Answer
Given below are two statements :Statement I: Aniline reacts with con. ...
Explanation of Statement I
Statement I asserts that aniline reacts with concentrated H₂SO₄ followed by heating to produce p-aminobenzenesulfonic acid. This is true because:
- Aniline (C₆H₅NH₂) undergoes sulfonation when treated with concentrated sulfuric acid.
- The reaction yields p-aminobenzenesulfonic acid, which is also known as sulfanilic acid.
- This compound gives a blood-red color in the Lassaigne's test, which is a qualitative analysis test for nitrogen-containing compounds.
Thus, Statement I is true.
Explanation of Statement II
Statement II discusses Friedel-Crafts reactions involving aniline and its interaction with AlCl₃. This statement is also true because:
- In Friedel-Crafts alkylation and acylation reactions, aniline can form a salt with AlCl₃.
- The nitrogen atom in aniline carries a lone pair of electrons and can coordinate with AlCl₃, leading to a positive charge on the nitrogen.
- This positive charge makes the aniline nitrogen a deactivating group, which reduces the nucleophilicity of the aromatic ring, thereby inhibiting further electrophilic aromatic substitution reactions.
Thus, Statement II is true.
Final Conclusion
In conclusion, both statements provide accurate information regarding the behavior of aniline in different chemical contexts:
- Statement I is true.
- Statement II is true.
Therefore, the correct answer is option D, as both statements are true.
Statement I asserts that aniline reacts with concentrated H₂SO₄ followed by heating to produce p-aminobenzenesulfonic acid. This is true because:
- Aniline (C₆H₅NH₂) undergoes sulfonation when treated with concentrated sulfuric acid.
- The reaction yields p-aminobenzenesulfonic acid, which is also known as sulfanilic acid.
- This compound gives a blood-red color in the Lassaigne's test, which is a qualitative analysis test for nitrogen-containing compounds.
Thus, Statement I is true.
Explanation of Statement II
Statement II discusses Friedel-Crafts reactions involving aniline and its interaction with AlCl₃. This statement is also true because:
- In Friedel-Crafts alkylation and acylation reactions, aniline can form a salt with AlCl₃.
- The nitrogen atom in aniline carries a lone pair of electrons and can coordinate with AlCl₃, leading to a positive charge on the nitrogen.
- This positive charge makes the aniline nitrogen a deactivating group, which reduces the nucleophilicity of the aromatic ring, thereby inhibiting further electrophilic aromatic substitution reactions.
Thus, Statement II is true.
Final Conclusion
In conclusion, both statements provide accurate information regarding the behavior of aniline in different chemical contexts:
- Statement I is true.
- Statement II is true.
Therefore, the correct answer is option D, as both statements are true.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer?
Question Description
Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer?.
Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Given below are two statements :Statement I: Aniline reacts with con. H2SO4followed by heating at 453-473 Kgives Paminobenzene sulphonic acid, which gives blood red colour in the Lassaignes test.Statement II: In Friedel - Crafts alkylation and acylation reactions, aniline forms salt with the AlCL3catalyst. Due to this, nitrogen of aniline acquires a positive charge and acts as a deactivating group.In the light of the above statements, choose the correct answer from the options given below :a)Statement I is false but statement II is trueb)Both statement I and statement II are falsec)Statement I is true but statement II is falsed)Both statement I and statement II are trueCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























