Class 12 Exam > Class 12 Questions > Select the correct observation about electrol...
Start Learning for Free
Select the correct observation about electrolysis.
- a)Electric current is used to drive a non-spontaneous reaction
- b)Ecell is positive and ΔG is negative
- c)Cations and anions are discharged at the cathode and anode respectively
- d)Overvoltage is responsible that a particular reaction takes place
Correct answer is option 'A,C,D'. Can you explain this answer?
Verified Answer
Select the correct observation about electrolysis.a)Electric current i...
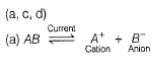
Thus, electric current is used to carry out a non-spontaneous reaction.
Thus. (a) is correct.
(b) Since reactions are reverse of electrochemical cell, hence
Thus. (a) is correct.
(b) Since reactions are reverse of electrochemical cell, hence
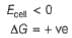
thus, (b) is incorrect
(c) cation goes to cathode and anion goes to anode and are discharged. Thus, correct
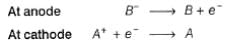
(d) some additional voltage called overvoltage is set up and thus other reactions also take place. thus correct
Most Upvoted Answer
Select the correct observation about electrolysis.a)Electric current i...
Observation about Electrolysis:
a) Electric current is used to drive a non-spontaneous reaction
Electrolysis is a process in which an electric current is used to drive a non-spontaneous chemical reaction. It involves the decomposition of a compound into its constituent elements or ions using an electric current. This process is used to produce various useful substances and is widely used in industries such as metallurgy, electroplating, and water electrolysis.
b) Ecell is positive and G is negative
The statement that "Ecell is positive and G is negative" is not correct. In electrolysis, the cell potential (Ecell) can be either positive or negative, depending on the specific reaction and the direction of electron flow. The sign of Ecell determines the direction of the reaction. If Ecell is positive, the reaction will proceed in the forward direction (electrons flow from anode to cathode), and if Ecell is negative, the reaction will proceed in the reverse direction (electrons flow from cathode to anode). The Gibbs free energy (G) for electrolysis is generally positive, as it requires energy input to drive a non-spontaneous reaction.
c) Cations and anions are discharged at the cathode and anode respectively
During electrolysis, cations (positively charged ions) are discharged at the cathode (negative electrode), and anions (negatively charged ions) are discharged at the anode (positive electrode). This is because opposite charges attract each other, and the cathode attracts cations while the anode attracts anions. This discharge of ions leads to the decomposition of the compound into its constituent elements.
d) Overvoltage is responsible for a particular reaction takes place
Overvoltage refers to the extra potential difference required to overcome the resistance at the electrode-electrolyte interface during electrolysis. It is responsible for a particular reaction to take place by ensuring that the desired reaction occurs at the electrode instead of other unwanted side reactions. Overvoltage depends on factors such as the nature of the electrode material, concentration of the electrolyte, and reaction conditions. By controlling the overvoltage, it is possible to selectively drive specific reactions during electrolysis.
In summary, the correct observations about electrolysis are:
- Electric current is used to drive a non-spontaneous reaction
- Cations and anions are discharged at the cathode and anode respectively
- Overvoltage is responsible for a particular reaction to take place.
a) Electric current is used to drive a non-spontaneous reaction
Electrolysis is a process in which an electric current is used to drive a non-spontaneous chemical reaction. It involves the decomposition of a compound into its constituent elements or ions using an electric current. This process is used to produce various useful substances and is widely used in industries such as metallurgy, electroplating, and water electrolysis.
b) Ecell is positive and G is negative
The statement that "Ecell is positive and G is negative" is not correct. In electrolysis, the cell potential (Ecell) can be either positive or negative, depending on the specific reaction and the direction of electron flow. The sign of Ecell determines the direction of the reaction. If Ecell is positive, the reaction will proceed in the forward direction (electrons flow from anode to cathode), and if Ecell is negative, the reaction will proceed in the reverse direction (electrons flow from cathode to anode). The Gibbs free energy (G) for electrolysis is generally positive, as it requires energy input to drive a non-spontaneous reaction.
c) Cations and anions are discharged at the cathode and anode respectively
During electrolysis, cations (positively charged ions) are discharged at the cathode (negative electrode), and anions (negatively charged ions) are discharged at the anode (positive electrode). This is because opposite charges attract each other, and the cathode attracts cations while the anode attracts anions. This discharge of ions leads to the decomposition of the compound into its constituent elements.
d) Overvoltage is responsible for a particular reaction takes place
Overvoltage refers to the extra potential difference required to overcome the resistance at the electrode-electrolyte interface during electrolysis. It is responsible for a particular reaction to take place by ensuring that the desired reaction occurs at the electrode instead of other unwanted side reactions. Overvoltage depends on factors such as the nature of the electrode material, concentration of the electrolyte, and reaction conditions. By controlling the overvoltage, it is possible to selectively drive specific reactions during electrolysis.
In summary, the correct observations about electrolysis are:
- Electric current is used to drive a non-spontaneous reaction
- Cations and anions are discharged at the cathode and anode respectively
- Overvoltage is responsible for a particular reaction to take place.

|
Explore Courses for Class 12 exam
|

|
Question Description
Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer?.
Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer?.
Solutions for Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer?, a detailed solution for Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer? has been provided alongside types of Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Select the correct observation about electrolysis.a)Electric current is used to drive a non-spontaneous reactionb)Ecell is positive and ΔG is negativec)Cations and anions are discharged at the cathode and anode respectivelyd)Overvoltage is responsible that a particular reaction takes placeCorrect answer is option 'A,C,D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























