MCAT Exam > MCAT Questions > Which of the following statements about atomi...
Start Learning for Free
Which of the following statements about atomic radii accurately describes the data below?
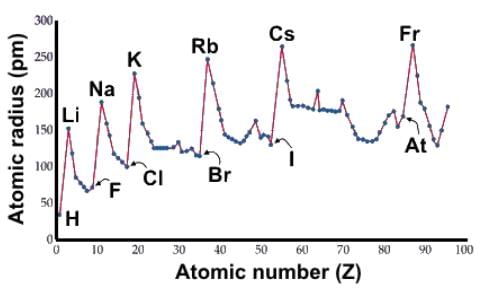
- a)The elements right after the d-block experience an expansion due to the repulsion created by the d electrons such that period 4 non metals are substantially larger than period 3 nonmetals.
- b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.
- c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.
- d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
Which of the following statements about atomic radii accurately descri...
Solution:
- The atomic size of transition metals remains relatively consistent across a period.
- This consistency is because the valence shell primarily involves the s orbital, which does not change much.
- As a result, the atomic radii of transition metals do not significantly increase or decrease across the group.

|
Explore Courses for MCAT exam
|

|
Question Description
Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer? for MCAT 2025 is part of MCAT preparation. The Question and answers have been prepared according to the MCAT exam syllabus. Information about Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for MCAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer?.
Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer? for MCAT 2025 is part of MCAT preparation. The Question and answers have been prepared according to the MCAT exam syllabus. Information about Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for MCAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer?.
Solutions for Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for MCAT.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
Here you can find the meaning of Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following statements about atomic radii accurately describes the data below?a)The elements right after thed-block experience an expansion due to the repulsion created by thed electrons such that period 4 non metals are substantially larger than period 3 nonmetals.b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice MCAT tests.

|
Explore Courses for MCAT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















