GMAT Exam > GMAT Questions > The graph shows how the solubility of various...
Start Learning for Free
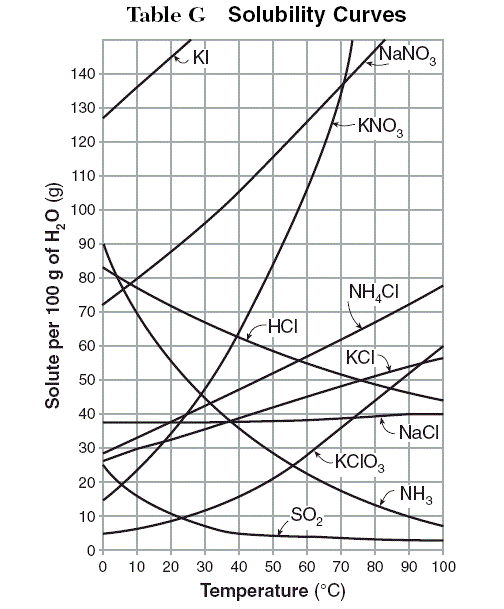
The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.
Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.
1. At 60°C, the most soluble of the chemicals is most likely__________.
2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.
Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.
1. At 60°C, the most soluble of the chemicals is most likely__________.
2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.
- a)1. SO2
2. KCl - b)1. KI
2. KClO3 - c)1. HCl
2. NH3 - d)1. SO2
2. NaCl - e)1. KClO32. NaNO3
Correct answer is option 'B'. Can you explain this answer?
Most Upvoted Answer
The graph shows how the solubility of various chemicals varies with te...
By following the gridline at 60°C, the visible line indicating the solubility of NaNO3 is highest on the graph. However, the line for KI extends above the scale shown on the graph. So, given the assumption that the curves continue in the same general shape beyond the plotted area, the information given indicates that KI is the most soluble at 60°C.
The correct answer is KI.
The vertical distance between the solubility curves for KClO3 and NH4Cl decreases as the temperature increases, thus the difference in solubility between KClO3 and NH4Cl decreases as the temperature increases. Assuming that the curves continue in the same general shape beyond the plotted area, the vertical distance between them will eventually be zero, thereby indicating that there is a temperature at which the two chemicals are equally soluble. By contrast, the solubility curves for KCl and NaCl have increasingly greater vertical distances from that of NH4Cl as the temperature increases. Thus the differences in the solubilities of either KCl or NaCl as compared to that of NH4Cl would continue to increase as the temperature increases.
The correct answer is KClO3.
The correct answer is KI.
The vertical distance between the solubility curves for KClO3 and NH4Cl decreases as the temperature increases, thus the difference in solubility between KClO3 and NH4Cl decreases as the temperature increases. Assuming that the curves continue in the same general shape beyond the plotted area, the vertical distance between them will eventually be zero, thereby indicating that there is a temperature at which the two chemicals are equally soluble. By contrast, the solubility curves for KCl and NaCl have increasingly greater vertical distances from that of NH4Cl as the temperature increases. Thus the differences in the solubilities of either KCl or NaCl as compared to that of NH4Cl would continue to increase as the temperature increases.
The correct answer is KClO3.

|
Explore Courses for GMAT exam
|

|
Question Description
The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer? for GMAT 2025 is part of GMAT preparation. The Question and answers have been prepared according to the GMAT exam syllabus. Information about The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for GMAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer?.
The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer? for GMAT 2025 is part of GMAT preparation. The Question and answers have been prepared according to the GMAT exam syllabus. Information about The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for GMAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer?.
Solutions for The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for GMAT.
Download more important topics, notes, lectures and mock test series for GMAT Exam by signing up for free.
Here you can find the meaning of The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer?, a detailed solution for The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.1. At 60°C, the most soluble of the chemicals is most likely__________.2. There is most likely a temperature above 100°C at which NH4Cl is exactly as soluble as ___________.a)1. SO22. KClb)1. KI2.KClO3c)1. HCl2.NH3d)1. SO22. NaCle)1. KClO32.NaNO3Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice GMAT tests.

|
Explore Courses for GMAT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.