Chemistry Exam > Chemistry Questions > The extent of π electron conjugation in ...
Start Learning for Free
The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:
- a)(i) > (iii) > (ii)
- b)(i) > (ii) > (iii)
- c)(iii) > (i) > (ii)
- d)(ii) ≈ (i) > (iii)
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
The extent of π electron conjugation in macrocyclic rings of (i) ...
Least conjugation is present in Vitamin B12 as one side of the corrin ring doesn’t have methylene linkages. In Heme, there is extensive conjugation of π electron (11 π ) as compared to that of chlorophyll.( 10π )
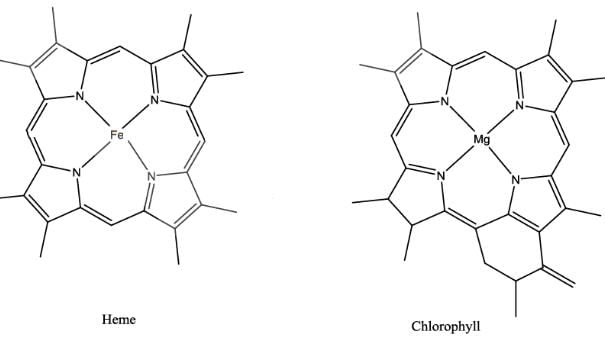
Most Upvoted Answer
The extent of π electron conjugation in macrocyclic rings of (i) ...
Heme have a phorphyring which have 11 conjugated bonds.... Coenzyme B12 have corin ring which have 6 conjugated bonds while chlorophyll have chlorine ring which have 10 conjugated bonds.. ... So extent of conjugation is directly proportional to the no of double bonds.. So order will be 1>3>2
Free Test
FREE
| Start Free Test |
Community Answer
The extent of π electron conjugation in macrocyclic rings of (i) ...
Explanation:
The extent of electron conjugation in macrocyclic rings can be determined by analyzing the aromaticity and the delocalization of electrons within the ring system. In this case, we are comparing the extent of electron conjugation in the macrocyclic rings of heme, coenzyme B12, and chlorophyll.
I. Heme:
Heme is a macrocyclic ring structure that consists of four pyrrole rings linked together by methene bridges. The central iron atom in heme is coordinated by a histidine residue, forming a porphyrin complex. The conjugated system of double bonds in the pyrrole rings gives heme its characteristic color and is responsible for its ability to bind and transport oxygen in hemoglobin.
II. Coenzyme B12:
Coenzyme B12, also known as cobalamin, is a macrocyclic ring structure that contains a cobalt ion at its center. The cobalt ion is coordinated by a dimethylbenzimidazole group and a 5,6-dimethylbenzimidazole group. The macrocyclic ring of coenzyme B12 contains multiple double bonds, which contribute to its redox properties and its role as a cofactor in enzymatic reactions.
III. Chlorophyll:
Chlorophyll is a macrocyclic ring structure that is responsible for the absorption of light in photosynthetic organisms. It consists of a porphyrin ring system with a magnesium ion at its center. The conjugated system of double bonds in the porphyrin ring allows chlorophyll to absorb light energy and transfer it to other molecules in the photosynthetic process.
Extent of Electron Conjugation:
Based on the structures and properties of heme, coenzyme B12, and chlorophyll, the extent of electron conjugation in their macrocyclic rings can be determined as follows:
(i) Heme: Heme has a highly conjugated system of double bonds due to the presence of four pyrrole rings. The delocalization of electrons in the conjugated system makes heme aromatic and highly conjugated.
(ii) Coenzyme B12: Coenzyme B12 also has a conjugated system of double bonds in its macrocyclic ring. However, the presence of the cobalt ion at the center of the ring disrupts the delocalization of electrons to some extent. Therefore, the extent of electron conjugation in coenzyme B12 is lower than that in heme.
(iii) Chlorophyll: Chlorophyll has a similar macrocyclic ring structure to heme, with a porphyrin ring and a central metal ion (magnesium). The conjugated system of double bonds in the porphyrin ring allows for a high extent of electron conjugation, making chlorophyll highly conjugated.
Conclusion:
Based on the extent of electron conjugation in their macrocyclic rings, the correct order is:
a) (i) Heme, (iii) Chlorophyll, (ii) Coenzyme B

|
Explore Courses for Chemistry exam
|

|
Question Description
The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer?.
The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer?.
Solutions for The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer?, a detailed solution for The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:a)(i) > (iii) > (ii)b)(i) > (ii) > (iii)c)(iii) > (i) > (ii)d)(ii) ≈ (i) > (iii)Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























