Chemistry Exam > Chemistry Questions > For the chemical reaction,The amount of X3 Y ...
Start Learning for Free
For the chemical reaction,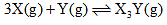
The amount of X3 Y at equilibrium is affected by
- a)Temperature and pressure
- b)Temperature only
- c)Pressure only
- d)Temperature, pressure and catalyst
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
For the chemical reaction,The amount of X3 Y at equilibrium is affecte...
There are 3 main factors that affect the equilibrium of a reaction:
- Concentration of reactants or products
- Temperature
- Pressure (Affects only gaseous reactions)
Most Upvoted Answer
For the chemical reaction,The amount of X3 Y at equilibrium is affecte...
Look count the no of moles both sides that is 4 on the rectant side and 1 on the product side the difference of moles is -3 hence pressure vary with the reaction quotient as inversely so increase or decrease of pressure will change the reaction quotient according to which it depends on whether it is greater or less than the equilibrium constant so it changes the direction also equlilibrium affect by the temperature depends on. whether the section is exothermic or endothermic so option is a
Free Test
FREE
| Start Free Test |
Community Answer
For the chemical reaction,The amount of X3 Y at equilibrium is affecte...
Eigther reaction would be endothermic or exothermic so temperature affects in both case and on second hand since there is change in concentration so pressure will definetly change euqilibrium.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer?
Question Description
For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer?.
For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer?.
Solutions for For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice For the chemical reaction,The amount of X3 Y at equilibrium is affected bya)Temperature and pressureb)Temperature onlyc)Pressure onlyd)Temperature, pressure and catalystCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















