Class 11 Exam > Class 11 Questions > Boric acid is an acid because its moleculea)c...
Start Learning for Free
Boric acid is an acid because its molecule
- a)contains replaceable H+ ion
- b)gives up a proton
- c)accepts OH– from water releasing proton
- d)combines with proton from water molecule
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Boric acid is an acid because its moleculea)contains replaceable H+ io...
Because of the small size of boron atom and presence of only six electrons in its valence shell, B(OH)3 accepts a pair of electrons from OH- ion of H20, releasing a proton.
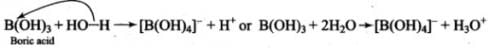
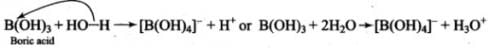
Most Upvoted Answer
Boric acid is an acid because its moleculea)contains replaceable H+ io...
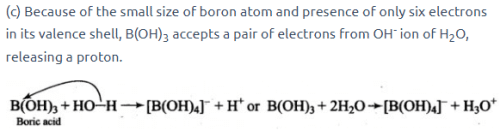
Free Test
FREE
| Start Free Test |
Community Answer
Boric acid is an acid because its moleculea)contains replaceable H+ io...
Understanding Boric Acid
Boric acid (H3BO3) is often categorized as a weak acid due to its unique behavior in aqueous solution. Its classification as an acid can be understood through its interaction with water.
Mechanism of Action
- Proton Acceptance: Boric acid primarily acts as a Lewis acid. It accepts hydroxide ions (OH-) from water, leading to the release of protons (H+).
- Formation of Tetrahydroxyborate Ion: When boric acid interacts with water, it forms the tetrahydroxyborate ion (B(OH)4-) and releases a proton into the solution. This reaction can be represented as:
H3BO3 + H2O ⇌ B(OH)4- + H+
- Acidic Properties: The release of protons into the solution is what gives boric acid its acidic characteristics, even though it does not release H+ ions in the traditional sense of strong acids.
Comparison with Other Acids
- Weak vs. Strong Acids: Unlike strong acids that fully dissociate in solution, boric acid partially dissociates, making it a weak acid. Its ability to accept hydroxide ions distinguishes it from typical Arrhenius acids that donate protons directly.
- Role of Water: The interaction with water is crucial, as it facilitates the acceptance of OH- ions, which is essential for the acid-base reaction.
Conclusion
In summary, boric acid classifies as an acid primarily because it accepts hydroxide ions from water, resulting in the release of protons. This unique mechanism of action highlights the complexity of acid behavior in chemistry.
Boric acid (H3BO3) is often categorized as a weak acid due to its unique behavior in aqueous solution. Its classification as an acid can be understood through its interaction with water.
Mechanism of Action
- Proton Acceptance: Boric acid primarily acts as a Lewis acid. It accepts hydroxide ions (OH-) from water, leading to the release of protons (H+).
- Formation of Tetrahydroxyborate Ion: When boric acid interacts with water, it forms the tetrahydroxyborate ion (B(OH)4-) and releases a proton into the solution. This reaction can be represented as:
H3BO3 + H2O ⇌ B(OH)4- + H+
- Acidic Properties: The release of protons into the solution is what gives boric acid its acidic characteristics, even though it does not release H+ ions in the traditional sense of strong acids.
Comparison with Other Acids
- Weak vs. Strong Acids: Unlike strong acids that fully dissociate in solution, boric acid partially dissociates, making it a weak acid. Its ability to accept hydroxide ions distinguishes it from typical Arrhenius acids that donate protons directly.
- Role of Water: The interaction with water is crucial, as it facilitates the acceptance of OH- ions, which is essential for the acid-base reaction.
Conclusion
In summary, boric acid classifies as an acid primarily because it accepts hydroxide ions from water, resulting in the release of protons. This unique mechanism of action highlights the complexity of acid behavior in chemistry.

|
Explore Courses for Class 11 exam
|

|
Question Description
Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer?.
Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Boric acid is an acid because its moleculea)contains replaceable H+ ionb)gives up a protonc)accepts OH–from water releasing protond)combines with proton from water moleculeCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























