Class 9 Exam > Class 9 Questions > In an experiment 1.288gm of compromise was ob...
Start Learning for Free
In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion?
Most Upvoted Answer
In an experiment 1.288gm of compromise was obtained from1.03gm of copp...
**The Law of Constant Proportions**
The law of constant proportions, also known as the law of definite proportions, states that a given chemical compound always contains the same elements in the same fixed proportion by mass. This means that regardless of the source or method of preparation, the ratio of the masses of the elements in a compound is always constant.
**Experimental Data**
Let's analyze the given experimental data to determine if it verifies the law of constant proportions.
**Experiment 1:**
- Mass of copper = 1.03 gm
- Mass of compound obtained = 1.288 gm
**Experiment 2:**
- Mass of copper = 2.938 gm
- Mass of compound obtained = 3.672 gm
**Calculations:**
To verify the law of constant proportions, we need to compare the ratio of the masses of copper and the compound obtained in both experiments.
**Experiment 1:**
- Mass ratio of copper to compound = 1.03 gm : 1.288 gm = 0.8
**Experiment 2:**
- Mass ratio of copper to compound = 2.938 gm : 3.672 gm = 0.8
From the calculations, we can see that the ratio of masses of copper to the compound obtained is the same in both experiments. This indicates that the law of constant proportions is being verified.
**Explanation:**
The law of constant proportions can be explained by the atomic theory. According to this theory, elements are composed of indivisible particles called atoms. These atoms combine in fixed ratios to form compounds. In a compound, the atoms are held together by chemical bonds.
The law of constant proportions suggests that the ratio of the masses of elements in a compound is determined by the ratio of their atoms. Since atoms are indivisible, they cannot be created or destroyed during a chemical reaction. Therefore, the ratio of the masses of elements in a compound remains constant.
In the given experiments, the ratio of the masses of copper and the compound obtained is the same, indicating that the same compound is being formed. This confirms the law of constant proportions.
**Conclusion:**
Based on the experimental data and calculations, it can be concluded that the figures verify the law of constant proportions. The consistent ratio of the masses of copper and the compound obtained in both experiments supports the idea that a given compound always contains the same elements in the same fixed proportion by mass.
The law of constant proportions, also known as the law of definite proportions, states that a given chemical compound always contains the same elements in the same fixed proportion by mass. This means that regardless of the source or method of preparation, the ratio of the masses of the elements in a compound is always constant.
**Experimental Data**
Let's analyze the given experimental data to determine if it verifies the law of constant proportions.
**Experiment 1:**
- Mass of copper = 1.03 gm
- Mass of compound obtained = 1.288 gm
**Experiment 2:**
- Mass of copper = 2.938 gm
- Mass of compound obtained = 3.672 gm
**Calculations:**
To verify the law of constant proportions, we need to compare the ratio of the masses of copper and the compound obtained in both experiments.
**Experiment 1:**
- Mass ratio of copper to compound = 1.03 gm : 1.288 gm = 0.8
**Experiment 2:**
- Mass ratio of copper to compound = 2.938 gm : 3.672 gm = 0.8
From the calculations, we can see that the ratio of masses of copper to the compound obtained is the same in both experiments. This indicates that the law of constant proportions is being verified.
**Explanation:**
The law of constant proportions can be explained by the atomic theory. According to this theory, elements are composed of indivisible particles called atoms. These atoms combine in fixed ratios to form compounds. In a compound, the atoms are held together by chemical bonds.
The law of constant proportions suggests that the ratio of the masses of elements in a compound is determined by the ratio of their atoms. Since atoms are indivisible, they cannot be created or destroyed during a chemical reaction. Therefore, the ratio of the masses of elements in a compound remains constant.
In the given experiments, the ratio of the masses of copper and the compound obtained is the same, indicating that the same compound is being formed. This confirms the law of constant proportions.
**Conclusion:**
Based on the experimental data and calculations, it can be concluded that the figures verify the law of constant proportions. The consistent ratio of the masses of copper and the compound obtained in both experiments supports the idea that a given compound always contains the same elements in the same fixed proportion by mass.
Community Answer
In an experiment 1.288gm of compromise was obtained from1.03gm of copp...
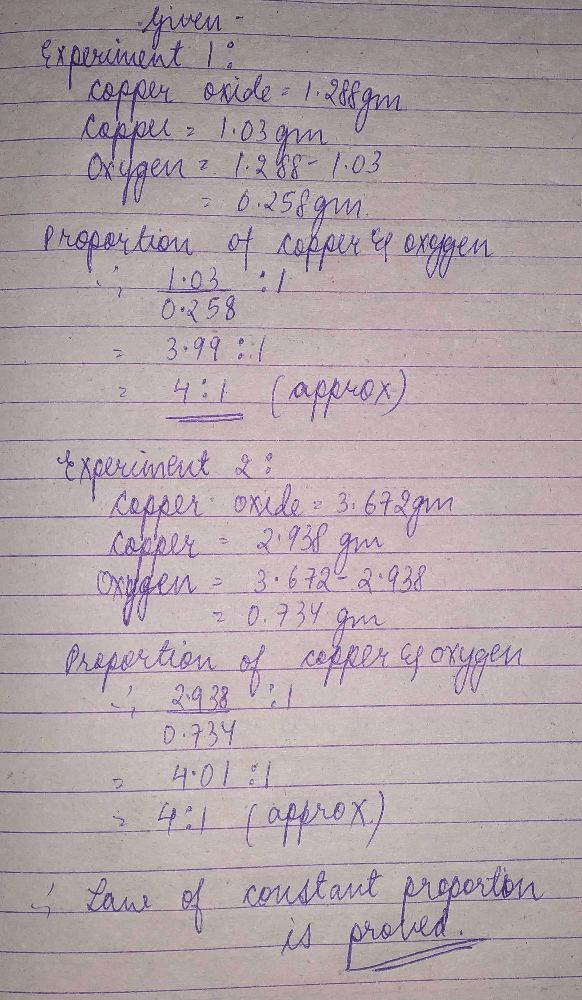
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion?
Question Description
In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion?.
In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion?.
Solutions for In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion? defined & explained in the simplest way possible. Besides giving the explanation of
In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion?, a detailed solution for In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion? has been provided alongside types of In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion? theory, EduRev gives you an
ample number of questions to practice In an experiment 1.288gm of compromise was obtained from1.03gm of copper. in other experiment 3.672gm of compromise gave a reduction of 2.938gm of copper. so that the figures verify the law of constant proportion? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























