Class 12 Exam > Class 12 Questions > When a radioactive nucleus emits a β ...
Start Learning for Free
When a radioactive nucleus emits a β – particle, the mass number of the atom
- a)Increases by one
- b)Decreases by one
- c)Remain the same
- d)Decreaseds by four
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
When a radioactive nucleus emits a β – particle, the mass n...
When a radioactive nucleus emits an alpha particle, the mass number of the atom remains the same.
Explanation:
- Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. There are several types of radioactive decay, including alpha decay, beta decay, and gamma decay.
- Alpha decay occurs when a radioactive nucleus emits an alpha particle, which consists of two protons and two neutrons. This emission reduces the atomic number and mass number of the parent nucleus.
- In alpha decay, the atomic number decreases by two because the emission of an alpha particle means the loss of two protons. The atomic number represents the number of protons in an atom, so when two protons are lost, the atomic number decreases.
- On the other hand, the mass number remains the same because the mass number represents the sum of protons and neutrons in an atom. Since the alpha particle contains two protons and two neutrons, when it is emitted, the mass number of the parent nucleus remains unchanged.
- For example, if we consider the alpha decay of uranium-238 (U-238), the parent nucleus emits an alpha particle (He-4) and transforms into thorium-234 (Th-234). In this decay process, the atomic number of U-238 decreases from 92 to 90, but the mass number remains the same at 238.
- This concept is consistent with the conservation of mass and charge. The total number of protons and neutrons in the nucleus, which is represented by the mass number, is conserved during alpha decay. However, the number of protons, which determines the atomic number and the chemical properties of an element, changes.
- It is important to note that the emission of an alpha particle is only one type of radioactive decay. Other types of decay, such as beta decay, can result in changes in both the atomic number and mass number of the parent nucleus.
Explanation:
- Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. There are several types of radioactive decay, including alpha decay, beta decay, and gamma decay.
- Alpha decay occurs when a radioactive nucleus emits an alpha particle, which consists of two protons and two neutrons. This emission reduces the atomic number and mass number of the parent nucleus.
- In alpha decay, the atomic number decreases by two because the emission of an alpha particle means the loss of two protons. The atomic number represents the number of protons in an atom, so when two protons are lost, the atomic number decreases.
- On the other hand, the mass number remains the same because the mass number represents the sum of protons and neutrons in an atom. Since the alpha particle contains two protons and two neutrons, when it is emitted, the mass number of the parent nucleus remains unchanged.
- For example, if we consider the alpha decay of uranium-238 (U-238), the parent nucleus emits an alpha particle (He-4) and transforms into thorium-234 (Th-234). In this decay process, the atomic number of U-238 decreases from 92 to 90, but the mass number remains the same at 238.
- This concept is consistent with the conservation of mass and charge. The total number of protons and neutrons in the nucleus, which is represented by the mass number, is conserved during alpha decay. However, the number of protons, which determines the atomic number and the chemical properties of an element, changes.
- It is important to note that the emission of an alpha particle is only one type of radioactive decay. Other types of decay, such as beta decay, can result in changes in both the atomic number and mass number of the parent nucleus.
Free Test
FREE
| Start Free Test |
Community Answer
When a radioactive nucleus emits a β – particle, the mass n...
A beta particle forms when a neutron changes the mass number stays the same.
Example
Carbon-14 decays into nitrogen-14 by emitting a beta particle. This is the balanced equation for the reaction:
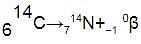
Notice that:
Example
Carbon-14 decays into nitrogen-14 by emitting a beta particle. This is the balanced equation for the reaction:
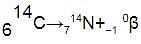
Notice that:
- the mass number of the nucleus stays the same, 14
- the atomic number of the nucleus increases by 1, from 6 to 7
- the numbers at the top and bottom give the same totals on both sides, as (7 - 1) = 6
Nitrogen nuclei have 7 protons, so their nuclear charge is +7. They have one more proton than carbon nuclei, which have 6 protons and so a nuclear charge of +6.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer?
Question Description
When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer?.
When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer?.
Solutions for When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When a radioactive nucleus emits a β – particle, the mass number of the atoma)Increases by oneb)Decreases by onec)Remain the samed)Decreaseds by fourCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















