Class 11 Exam > Class 11 Questions > Hybridization ofC2andC3ofH3C−−CH=...
Start Learning for Free
Hybridization of C2and C3of H3C −− CH = C = CH −− CH3are
- a)Sp, Sp3
- b)Sp2, Sp2
- c)Sp2, Sp
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area...
The first carbon atom forms four sigma bonds, three with Hydrogen and one with carbon. So, the carbon here is sp3 hybridised.The second carbon atom forms three sigma bonds and one pi bond. The three sigma bonds can be possible only when carbon is sp2 hybridised. The fourth electron forms a pi overlap with an electron from third carbon atom.
Carbon atom 3 forms two sigma bonds and is sp hybridised. The two p electrons form a pi bond with p electrons of the neighbouring carbon atoms.Carbon atom 4 is similar to carbon 2, forms 3 sigma bonds and is sp2 hybridised.
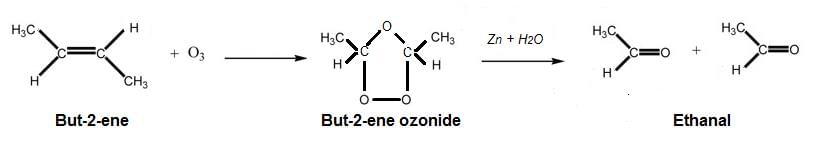
As the aldehyde formed has molar mass of 44u, so the aldehyde is acetaldehyde or ethanal. The alkene that gives rise to ethanal, is but-2-ene. Ozonolysis leads to breaking the alkene molecule into two molecules at the double bond. As only one product, ethanal is formed, two carbon atoms surround the two sides of the double bond.
Free Test
FREE
| Start Free Test |
Community Answer
Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area...
To determine the hybridization of carbon atoms C2 and C3 in H3C, we need to first draw the Lewis structure of the molecule.
H3C can be represented as:
H - C - C - H
|
H
In this structure, carbon atom C2 is bonded to three hydrogen atoms and one carbon atom (C3), while carbon atom C3 is bonded to two hydrogen atoms and one carbon atom (C2).
Now, let's determine the hybridization of C2 and C3.
To do this, we count the number of regions of electron density around each carbon atom. This includes the number of bonded atoms and lone pairs.
For C2:
- C2 is bonded to three hydrogen atoms and one carbon atom (C3). This gives a total of 4 regions of electron density.
For C3:
- C3 is bonded to two hydrogen atoms and one carbon atom (C2). This also gives a total of 3 regions of electron density.
To determine the hybridization, we use the following guidelines:
- 2 regions of electron density: sp hybridization
- 3 regions of electron density: sp2 hybridization
- 4 regions of electron density: sp3 hybridization
Based on this, we can conclude that carbon atom C2 has sp3 hybridization (since it has 4 regions of electron density), and carbon atom C3 has sp2 hybridization (since it has 3 regions of electron density).
H3C can be represented as:
H - C - C - H
|
H
In this structure, carbon atom C2 is bonded to three hydrogen atoms and one carbon atom (C3), while carbon atom C3 is bonded to two hydrogen atoms and one carbon atom (C2).
Now, let's determine the hybridization of C2 and C3.
To do this, we count the number of regions of electron density around each carbon atom. This includes the number of bonded atoms and lone pairs.
For C2:
- C2 is bonded to three hydrogen atoms and one carbon atom (C3). This gives a total of 4 regions of electron density.
For C3:
- C3 is bonded to two hydrogen atoms and one carbon atom (C2). This also gives a total of 3 regions of electron density.
To determine the hybridization, we use the following guidelines:
- 2 regions of electron density: sp hybridization
- 3 regions of electron density: sp2 hybridization
- 4 regions of electron density: sp3 hybridization
Based on this, we can conclude that carbon atom C2 has sp3 hybridization (since it has 4 regions of electron density), and carbon atom C3 has sp2 hybridization (since it has 3 regions of electron density).

|
Explore Courses for Class 11 exam
|

|
Question Description
Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer?.
Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Hybridization ofC2andC3ofH3C−−CH=C=CH−−CH3area)Sp,Sp3b)Sp2,Sp2c)Sp2, Spd)None of the aboveCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















