Class 11 Exam > Class 11 Questions > Photochemical chlorination of alkane is initi...
Start Learning for Free
Photochemical chlorination of alkane is initiated by process called:
- a)Homolysis
- b)Pyrolysis
- c)Substitution
- d)Peroxidation
Correct answer is option 'A'. Can you explain this answer?
Most Upvoted Answer
Photochemical chlorination of alkane is initiated by process called:a)...
Photochemical chlorination of alkane take place by free radical mechanism which are possible by Homolysis of C - C bond
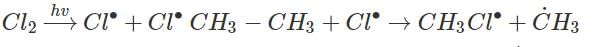
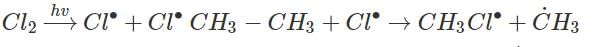
Free Test
FREE
| Start Free Test |
Community Answer
Photochemical chlorination of alkane is initiated by process called:a)...
**Homolysis: The Initiation Step of Photochemical Chlorination**
The photochemical chlorination of alkane is initiated by a process called homolysis. Homolysis refers to the breaking of a covalent bond, resulting in the formation of two radicals, each carrying one electron. In the case of photochemical chlorination, the bond that undergoes homolysis is the carbon-hydrogen (C-H) bond in the alkane.
**The Process of Photochemical Chlorination**
Photochemical chlorination is a reaction that involves the substitution of a hydrogen atom in an alkane with a chlorine atom. It occurs under the influence of sunlight or ultraviolet (UV) radiation. The reaction proceeds through a series of steps, including initiation, propagation, and termination.
**Initiation: Homolysis of Chlorine**
The initiation step involves the homolysis of a chlorine molecule (Cl2) to generate chlorine radicals (Cl•). The homolytic cleavage of the Cl-Cl bond occurs when the molecule absorbs energy from sunlight or UV radiation. The absorbed energy promotes one of the chlorine atoms to a higher energy level, resulting in the formation of Cl• radicals.
Cl2 → 2 Cl•
**Propagation: Radical Chain Reaction**
Once the chlorine radicals are formed, they react with the alkane molecule to initiate a radical chain reaction. The reaction proceeds through the following steps:
1. Chlorine radical abstraction: A chlorine radical reacts with the alkane, abstracting a hydrogen atom from one of the carbon atoms. This process forms a hydrogen chloride (HCl) molecule and an alkyl radical.
Cl• + RH → HCl + R•
2. Chlorine radical regeneration: The alkyl radical formed in the previous step then reacts with another chlorine molecule, regenerating the chlorine radical and forming a chlorinated alkane.
R• + Cl2 → RCl + Cl•
**Termination: Radical Combination or Disproportionation**
The radical chain reaction can continue until all the hydrogen atoms in the alkane are replaced by chlorine atoms. However, it can also be terminated through two processes:
1. Radical combination: Two alkyl radicals can combine to form a covalent bond, eliminating the unpaired electrons and producing a stable molecule.
R• + R• → R-R
2. Radical disproportionation: A radical can react with a molecule of the same kind, resulting in the transfer of a hydrogen atom and electron to form a stable molecule and a new radical species.
R• + R' • → R-R' + H•
**In conclusion**, the photochemical chlorination of alkane is initiated by the process of homolysis. This process involves the homolytic cleavage of a chlorine molecule to generate chlorine radicals, which then react with the alkane to initiate a radical chain reaction. The reaction proceeds through initiation, propagation, and termination steps, ultimately resulting in the substitution of hydrogen atoms with chlorine atoms in the alkane.
The photochemical chlorination of alkane is initiated by a process called homolysis. Homolysis refers to the breaking of a covalent bond, resulting in the formation of two radicals, each carrying one electron. In the case of photochemical chlorination, the bond that undergoes homolysis is the carbon-hydrogen (C-H) bond in the alkane.
**The Process of Photochemical Chlorination**
Photochemical chlorination is a reaction that involves the substitution of a hydrogen atom in an alkane with a chlorine atom. It occurs under the influence of sunlight or ultraviolet (UV) radiation. The reaction proceeds through a series of steps, including initiation, propagation, and termination.
**Initiation: Homolysis of Chlorine**
The initiation step involves the homolysis of a chlorine molecule (Cl2) to generate chlorine radicals (Cl•). The homolytic cleavage of the Cl-Cl bond occurs when the molecule absorbs energy from sunlight or UV radiation. The absorbed energy promotes one of the chlorine atoms to a higher energy level, resulting in the formation of Cl• radicals.
Cl2 → 2 Cl•
**Propagation: Radical Chain Reaction**
Once the chlorine radicals are formed, they react with the alkane molecule to initiate a radical chain reaction. The reaction proceeds through the following steps:
1. Chlorine radical abstraction: A chlorine radical reacts with the alkane, abstracting a hydrogen atom from one of the carbon atoms. This process forms a hydrogen chloride (HCl) molecule and an alkyl radical.
Cl• + RH → HCl + R•
2. Chlorine radical regeneration: The alkyl radical formed in the previous step then reacts with another chlorine molecule, regenerating the chlorine radical and forming a chlorinated alkane.
R• + Cl2 → RCl + Cl•
**Termination: Radical Combination or Disproportionation**
The radical chain reaction can continue until all the hydrogen atoms in the alkane are replaced by chlorine atoms. However, it can also be terminated through two processes:
1. Radical combination: Two alkyl radicals can combine to form a covalent bond, eliminating the unpaired electrons and producing a stable molecule.
R• + R• → R-R
2. Radical disproportionation: A radical can react with a molecule of the same kind, resulting in the transfer of a hydrogen atom and electron to form a stable molecule and a new radical species.
R• + R' • → R-R' + H•
**In conclusion**, the photochemical chlorination of alkane is initiated by the process of homolysis. This process involves the homolytic cleavage of a chlorine molecule to generate chlorine radicals, which then react with the alkane to initiate a radical chain reaction. The reaction proceeds through initiation, propagation, and termination steps, ultimately resulting in the substitution of hydrogen atoms with chlorine atoms in the alkane.

|
Explore Courses for Class 11 exam
|

|
Question Description
Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer?.
Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Photochemical chlorination of alkane is initiated by process called:a)Homolysisb)Pyrolysisc)Substitutiond)PeroxidationCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















