Mechanical Engineering Exam > Mechanical Engineering Questions > If a closed system is undergoing an irreversi...
Start Learning for Free
If a closed system is undergoing an irreversible process, the entropy of the system
- a)Must increase
- b)Always remains constant
- c)Must decrease
- d)Can increase, decrease or remain constant
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
If a closed system is undergoing an irreversible process, the entropy ...
If a closed system is undergoing an irreversible process, the change in entropy of the system is given by
dS > 0, or dS = 0, or dS < 0
In an irreversible process in which heat is removed from the system then its entropy can decrease.
The entropy of a closed system is given by
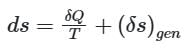
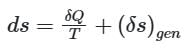
When the process is irreversible then entropy generation in the system (δs)gen is always positive, the heat transfer will decide whether the entropy will increase or decrease.
When heat is added to the system
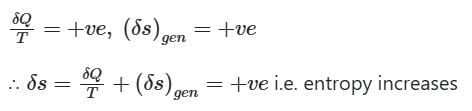
When Heat is removed from the system

When the process is adiabatic, dQ = 0,
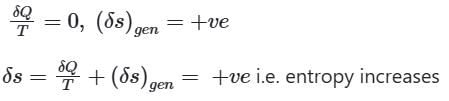
∴ when a closed system is undergoing an irreversible process the entropy may increase, decrease or remain constant.
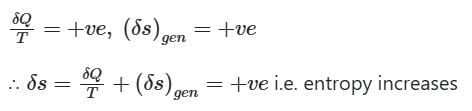
When Heat is removed from the system

When the process is adiabatic, dQ = 0,
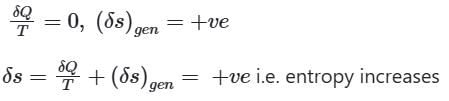
∴ when a closed system is undergoing an irreversible process the entropy may increase, decrease or remain constant.
Most Upvoted Answer
If a closed system is undergoing an irreversible process, the entropy ...
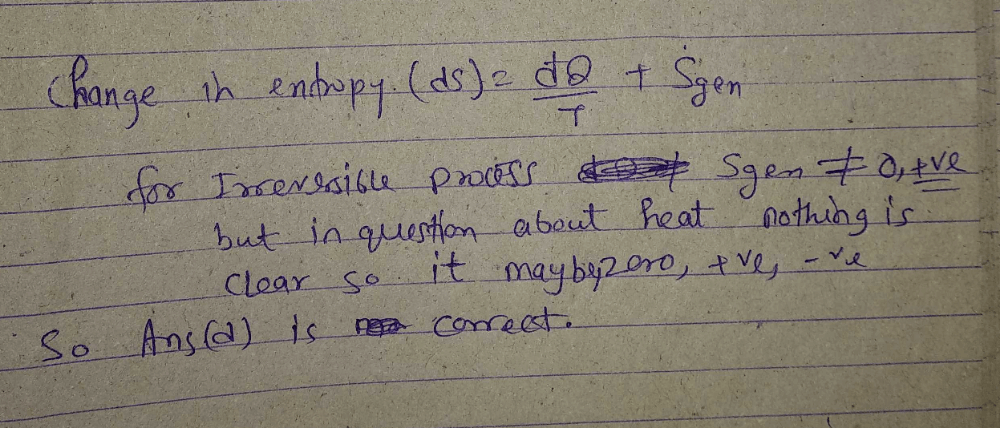
Free Test
FREE
| Start Free Test |
Community Answer
If a closed system is undergoing an irreversible process, the entropy ...
Irreversible Processes and Entropy
Irreversible Processes
• In a closed system, if the process takes place in such a way that it cannot be reversed, it is called an irreversible process.
• An irreversible process is characterized by the fact that it is not possible to restore the system and its surroundings to their initial states by means of infinitesimal changes.
• Examples of irreversible processes include heat flow from a hot body to a cold body, mixing of two gases, and expansion of a gas into a vacuum.
Entropy
• Entropy is a measure of the disorder or randomness of a system.
• It is a thermodynamic property that is related to the number of possible ways in which a system can be arranged.
• The entropy of a closed system can be increased by irreversible processes.
• The change in entropy of a system undergoing an irreversible process is given by the formula ΔS = Qrev/T, where Qrev is the heat transferred in a reversible process and T is the temperature at which the process takes place.
Answer
• If a closed system is undergoing an irreversible process, the entropy of the system can increase, decrease or remain constant.
• This is because the change in entropy of a system undergoing an irreversible process depends on the nature of the process and the initial and final states of the system.
• For example, if a gas is allowed to expand into a vacuum irreversibly, its entropy will increase.
• On the other hand, if a gas is compressed irreversibly, its entropy will decrease.
• If a system undergoes an irreversible process that does not involve any heat transfer, its entropy may remain constant.
• Therefore, it is not correct to say that the entropy of a system undergoing an irreversible process must increase.
Irreversible Processes
• In a closed system, if the process takes place in such a way that it cannot be reversed, it is called an irreversible process.
• An irreversible process is characterized by the fact that it is not possible to restore the system and its surroundings to their initial states by means of infinitesimal changes.
• Examples of irreversible processes include heat flow from a hot body to a cold body, mixing of two gases, and expansion of a gas into a vacuum.
Entropy
• Entropy is a measure of the disorder or randomness of a system.
• It is a thermodynamic property that is related to the number of possible ways in which a system can be arranged.
• The entropy of a closed system can be increased by irreversible processes.
• The change in entropy of a system undergoing an irreversible process is given by the formula ΔS = Qrev/T, where Qrev is the heat transferred in a reversible process and T is the temperature at which the process takes place.
Answer
• If a closed system is undergoing an irreversible process, the entropy of the system can increase, decrease or remain constant.
• This is because the change in entropy of a system undergoing an irreversible process depends on the nature of the process and the initial and final states of the system.
• For example, if a gas is allowed to expand into a vacuum irreversibly, its entropy will increase.
• On the other hand, if a gas is compressed irreversibly, its entropy will decrease.
• If a system undergoes an irreversible process that does not involve any heat transfer, its entropy may remain constant.
• Therefore, it is not correct to say that the entropy of a system undergoing an irreversible process must increase.

|
Explore Courses for Mechanical Engineering exam
|

|
Question Description
If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer?.
If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer?.
Solutions for If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice If a closed system is undergoing an irreversible process, the entropy of the systema)Must increaseb)Always remains constantc)Must decreased)Can increase, decrease or remain constantCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























