Class 12 Exam > Class 12 Questions > The minimum amount of energy required by the ...
Start Learning for Free
The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is called
- a)Threshold energy
- b)Potential energy
- c)Internal energy
- d)Activation energy
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
The minimum amount of energy required by the reacting molecules at the...
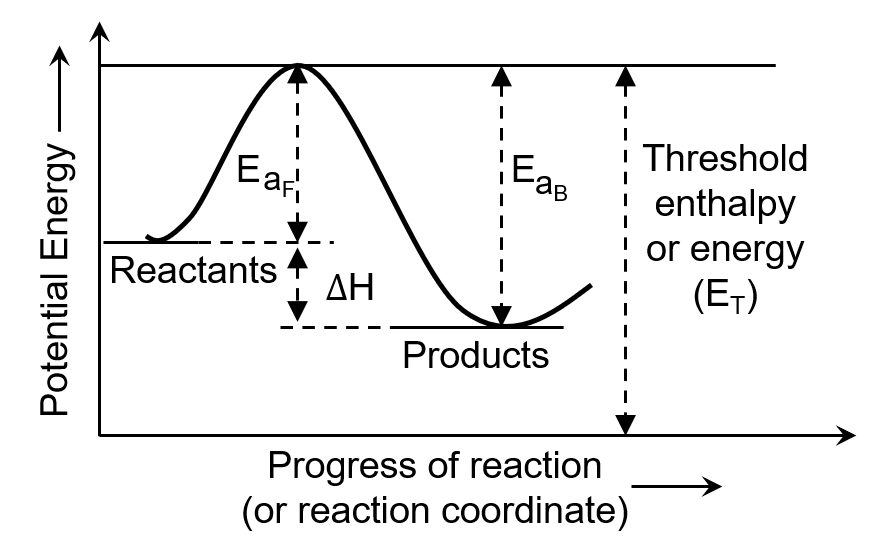
The minimum energy that the colliding molecules must possess for the chemical reaction to occur is known as threshold energy.
The extra energy required by a reactant to participate in a reaction is called activation energy.
Most Upvoted Answer
The minimum amount of energy required by the reacting molecules at the...
Threshold Energy for Effective Collisions
Threshold energy is the minimum energy required by the reacting molecules at the time of collisions in order to produce effective collisions. In other words, it is the minimum amount of energy that must be attained by colliding particles for a chemical reaction to occur.
Activation Energy
The minimum energy required by the reacting molecules to undergo a chemical reaction is called activation energy. It is the energy required to break the chemical bonds in the reactants and form new bonds in the products. Activation energy is the energy barrier that must be overcome for a reaction to occur.
Importance of Activation Energy
Activation energy is an important concept in chemistry because it determines the rate of a chemical reaction. The higher the activation energy, the slower the reaction will be. Conversely, the lower the activation energy, the faster the reaction will be.
Factors Affecting Activation Energy
The activation energy of a chemical reaction depends on several factors, including:
- The nature of the reactants
- The concentration of the reactants
- The temperature of the reaction
- The presence of a catalyst
Conclusion
In summary, activation energy is the minimum energy required by the reacting molecules to undergo a chemical reaction. Threshold energy is the minimum energy required by the colliding particles to produce effective collisions. Both activation energy and threshold energy are important concepts in chemistry, as they determine the rate of a chemical reaction.
Threshold energy is the minimum energy required by the reacting molecules at the time of collisions in order to produce effective collisions. In other words, it is the minimum amount of energy that must be attained by colliding particles for a chemical reaction to occur.
Activation Energy
The minimum energy required by the reacting molecules to undergo a chemical reaction is called activation energy. It is the energy required to break the chemical bonds in the reactants and form new bonds in the products. Activation energy is the energy barrier that must be overcome for a reaction to occur.
Importance of Activation Energy
Activation energy is an important concept in chemistry because it determines the rate of a chemical reaction. The higher the activation energy, the slower the reaction will be. Conversely, the lower the activation energy, the faster the reaction will be.
Factors Affecting Activation Energy
The activation energy of a chemical reaction depends on several factors, including:
- The nature of the reactants
- The concentration of the reactants
- The temperature of the reaction
- The presence of a catalyst
Conclusion
In summary, activation energy is the minimum energy required by the reacting molecules to undergo a chemical reaction. Threshold energy is the minimum energy required by the colliding particles to produce effective collisions. Both activation energy and threshold energy are important concepts in chemistry, as they determine the rate of a chemical reaction.
Free Test
FREE
| Start Free Test |
Community Answer
The minimum amount of energy required by the reacting molecules at the...
However, if energy is supplied in the form of heat, light etc, the reactant molecules absorb this energy becomes equal to or greater than threshold value. Hence, they start change into products. Evidently, less is the activation energy, faster is the reaction or greater is the activation energy, slower is the reaction.

|
Explore Courses for Class 12 exam
|

|
Question Description
The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer?.
The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer?.
Solutions for The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is calleda)Threshold energyb)Potential energyc)Internal energyd)Activation energyCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















