Class 11 Exam > Class 11 Questions > In a free radical reaction, free radicals are...
Start Learning for Free
In a free radical reaction, free radicals are formed at
- a)initiation step
- b)propagation step
- c)termination step
- d)both A and B
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
In a free radical reaction, free radicals are formed ata)initiation st...
Once a reactive free radical is generated, it can react with stable molecules to form new free radicals. These new free radicals go on to generate yet more free radicals, and so on. Propagation steps often involve hydrogen abstraction or addition of the radical to double bonds.
View all questions of this test
Most Upvoted Answer
In a free radical reaction, free radicals are formed ata)initiation st...
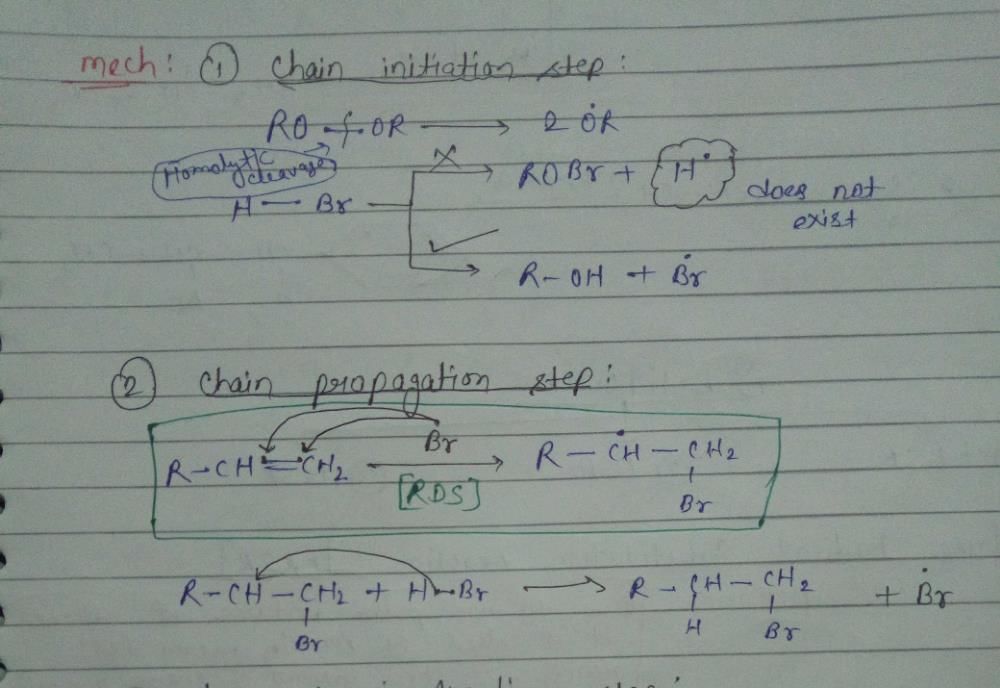
Initiation Step:
In a free radical reaction, the initiation step is the first step where free radicals are formed. It involves the breaking of a covalent bond to generate two highly reactive free radicals. This bond can be homolytically cleaved, meaning that each atom in the bond receives one of the shared electrons, resulting in the formation of two radicals.
Propagation Step:
The propagation step is the subsequent step in a free radical reaction where the newly formed free radicals react with other molecules to generate additional free radicals. This step occurs in a chain reaction mechanism where the free radicals generated in the initiation step react with the reactant molecules to form new radicals and product molecules.
In this step, the free radicals react with stable molecules, abstracting a hydrogen atom or an electron from the molecule, resulting in the formation of a new free radical and a new molecule with an unpaired electron. This new free radical can then react with another molecule, continuing the chain reaction.
Termination Step:
The termination step is the final step in a free radical reaction where the free radicals are consumed or deactivated, leading to the termination of the chain reaction. This step involves the combination of two free radicals to form a stable molecule, which no longer participates in the reaction.
In the termination step, two free radicals collide and their unpaired electrons pair up, forming a covalent bond. This process removes the unpaired electrons and stabilizes the system. The resulting molecule is no longer a free radical and does not participate further in the reaction.
Explanation:
In a free radical reaction, free radicals are formed in both the initiation and propagation steps. During the initiation step, a covalent bond is broken, generating two free radicals. These free radicals then participate in the propagation step, where they react with other molecules, generating more free radicals.
Therefore, the correct answer is option 'D' - both the initiation and propagation steps involve the formation of free radicals. The termination step, on the other hand, involves the combination of free radicals and leads to the termination of the reaction.
In a free radical reaction, the initiation step is the first step where free radicals are formed. It involves the breaking of a covalent bond to generate two highly reactive free radicals. This bond can be homolytically cleaved, meaning that each atom in the bond receives one of the shared electrons, resulting in the formation of two radicals.
Propagation Step:
The propagation step is the subsequent step in a free radical reaction where the newly formed free radicals react with other molecules to generate additional free radicals. This step occurs in a chain reaction mechanism where the free radicals generated in the initiation step react with the reactant molecules to form new radicals and product molecules.
In this step, the free radicals react with stable molecules, abstracting a hydrogen atom or an electron from the molecule, resulting in the formation of a new free radical and a new molecule with an unpaired electron. This new free radical can then react with another molecule, continuing the chain reaction.
Termination Step:
The termination step is the final step in a free radical reaction where the free radicals are consumed or deactivated, leading to the termination of the chain reaction. This step involves the combination of two free radicals to form a stable molecule, which no longer participates in the reaction.
In the termination step, two free radicals collide and their unpaired electrons pair up, forming a covalent bond. This process removes the unpaired electrons and stabilizes the system. The resulting molecule is no longer a free radical and does not participate further in the reaction.
Explanation:
In a free radical reaction, free radicals are formed in both the initiation and propagation steps. During the initiation step, a covalent bond is broken, generating two free radicals. These free radicals then participate in the propagation step, where they react with other molecules, generating more free radicals.
Therefore, the correct answer is option 'D' - both the initiation and propagation steps involve the formation of free radicals. The termination step, on the other hand, involves the combination of free radicals and leads to the termination of the reaction.
Free Test
FREE
| Start Free Test |
Community Answer
In a free radical reaction, free radicals are formed ata)initiation st...

|
Explore Courses for Class 11 exam
|

|
Question Description
In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer?.
In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer?.
Solutions for In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In a free radical reaction, free radicals are formed ata)initiation stepb)propagation stepc)termination stepd)both A and BCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















