Class 11 Exam > Class 11 Questions > Please tell structure of NaNC.and type of bon...
Start Learning for Free
Please tell structure of NaNC.and type of bonds in it.?
Most Upvoted Answer
Please tell structure of NaNC.and type of bonds in it.?
NaNC or Sodium Nitrite is an ionic compound that has a specific chemical structure and types of bonds. The structure and bond types can be explained as follows:
Structure:
- NaNC has a crystalline structure.
- It consists of sodium cations (Na+) and nitrite anions (NO2-).
- The nitrite anion has a trigonal planar shape, with the nitrogen atom at the center and two oxygen atoms at either end.
- The sodium cation is surrounded by six nitrite anions, forming an octahedral coordination complex.
- The chemical formula of NaNC is NaNO2.
Types of Bonds:
- The bond between sodium cation and nitrite anion is an ionic bond.
- In an ionic bond, electrons are transferred from one atom to another, resulting in the formation of charged ions that are attracted to each other by electrostatic forces.
- Sodium has a single valence electron in its outermost shell, while nitrite has two lone pairs of electrons on the nitrogen atom and a single bond with one oxygen atom.
- Sodium transfers its valence electron to one of the oxygen atoms of the nitrite anion, forming a sodium cation and a nitrite anion with a negative charge.
- The electrostatic attraction between the oppositely charged ions results in the formation of an ionic bond.
In conclusion, NaNC has a crystalline structure consisting of sodium cations and nitrite anions, forming an octahedral coordination complex. The bond between sodium cation and nitrite anion is an ionic bond, which results from the transfer of electrons from sodium to nitrite and the electrostatic attraction between oppositely charged ions.
Structure:
- NaNC has a crystalline structure.
- It consists of sodium cations (Na+) and nitrite anions (NO2-).
- The nitrite anion has a trigonal planar shape, with the nitrogen atom at the center and two oxygen atoms at either end.
- The sodium cation is surrounded by six nitrite anions, forming an octahedral coordination complex.
- The chemical formula of NaNC is NaNO2.
Types of Bonds:
- The bond between sodium cation and nitrite anion is an ionic bond.
- In an ionic bond, electrons are transferred from one atom to another, resulting in the formation of charged ions that are attracted to each other by electrostatic forces.
- Sodium has a single valence electron in its outermost shell, while nitrite has two lone pairs of electrons on the nitrogen atom and a single bond with one oxygen atom.
- Sodium transfers its valence electron to one of the oxygen atoms of the nitrite anion, forming a sodium cation and a nitrite anion with a negative charge.
- The electrostatic attraction between the oppositely charged ions results in the formation of an ionic bond.
In conclusion, NaNC has a crystalline structure consisting of sodium cations and nitrite anions, forming an octahedral coordination complex. The bond between sodium cation and nitrite anion is an ionic bond, which results from the transfer of electrons from sodium to nitrite and the electrostatic attraction between oppositely charged ions.
Community Answer
Please tell structure of NaNC.and type of bonds in it.?
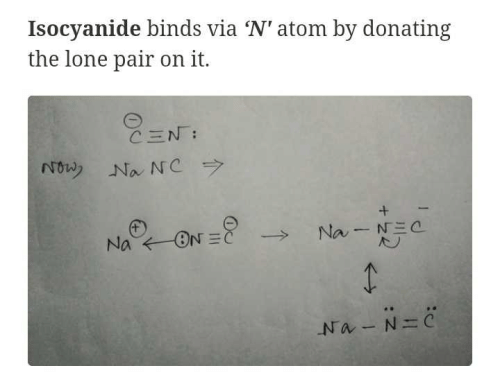

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Question Description
Please tell structure of NaNC.and type of bonds in it.? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Please tell structure of NaNC.and type of bonds in it.? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Please tell structure of NaNC.and type of bonds in it.?.
Please tell structure of NaNC.and type of bonds in it.? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Please tell structure of NaNC.and type of bonds in it.? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Please tell structure of NaNC.and type of bonds in it.?.
Solutions for Please tell structure of NaNC.and type of bonds in it.? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Please tell structure of NaNC.and type of bonds in it.? defined & explained in the simplest way possible. Besides giving the explanation of
Please tell structure of NaNC.and type of bonds in it.?, a detailed solution for Please tell structure of NaNC.and type of bonds in it.? has been provided alongside types of Please tell structure of NaNC.and type of bonds in it.? theory, EduRev gives you an
ample number of questions to practice Please tell structure of NaNC.and type of bonds in it.? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















