Class 11 Exam > Class 11 Questions > Among the following compounds the one that is...
Start Learning for Free
Among the following compounds the one that is most reactive towards electrophilic nitration is
- a)Benzene
- b)Toluene
- c)Benzoic Acid
- d)Nitrobenzene
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Among the following compounds the one that is most reactive towards el...
The presence of electron releasing group like -R, -OH etc., increases the electron density at o/p position and thus, makes the benzene ring more reactive (at(o/p position) towards electrophile. On the other hand, electron withdrawing group like-COOH,-NO2 etc. If present, reduces electron density and thus, reduces the activity benzene nucleus towards electrophile.Thus, the order of the given compounds towards electrophilic nitration is
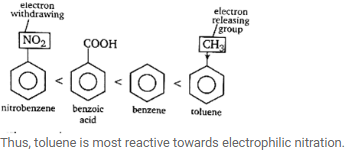
View all questions of this test

Most Upvoted Answer
Among the following compounds the one that is most reactive towards el...
Introduction:
Electrophilic nitration is a chemical reaction in which a nitro group (-NO2) is introduced into a molecule. This reaction is commonly used to synthesize nitroaromatic compounds, which are important intermediates in the production of dyes, pharmaceuticals, and explosives. The reactivity of a compound towards electrophilic nitration depends on its ability to stabilize the positive charge that forms during the reaction.
Explanation:
In the given options, the most reactive compound towards electrophilic nitration is toluene (option B). Let's understand why toluene is more reactive compared to the other options.
Benzene (option A):
Benzene is a highly stable aromatic compound due to its delocalized pi-electron system. The aromaticity of benzene makes it less reactive towards electrophilic substitution reactions like nitration. The delocalization of electrons in the benzene ring helps stabilize the positive charge formed during the nitration reaction, making it less likely to proceed.
Benzoic Acid (option C):
Benzoic acid is a derivative of benzene in which a carboxylic acid group (-COOH) is attached to the aromatic ring. The presence of the electron-withdrawing carboxylic acid group reduces the electron density on the benzene ring, making it moderately reactive towards electrophilic nitration compared to benzene. However, the electron-withdrawing effect of the carboxylic acid group still hinders the reaction to some extent.
Nitrobenzene (option D):
Nitrobenzene is a compound in which a nitro group (-NO2) is already attached to the benzene ring. The presence of the nitro group strongly withdraws electron density from the ring, making nitrobenzene less reactive towards electrophilic nitration. The electron-withdrawing effect of the nitro group deactivates the ring towards further electrophilic substitution reactions.
Toluene (option B):
Toluene is a compound in which a methyl group (-CH3) is attached to the benzene ring. The methyl group is electron-donating, meaning it increases the electron density on the benzene ring. This electron-donating effect makes toluene more reactive towards electrophilic nitration compared to benzene, benzoic acid, and nitrobenzene. The increased electron density on the ring enhances the nucleophilic attack on the electrophilic nitronium ion (NO2+) formed during the nitration reaction, facilitating the reaction.
Therefore, among the given options, toluene is the most reactive compound towards electrophilic nitration due to the electron-donating effect of the methyl group.
Electrophilic nitration is a chemical reaction in which a nitro group (-NO2) is introduced into a molecule. This reaction is commonly used to synthesize nitroaromatic compounds, which are important intermediates in the production of dyes, pharmaceuticals, and explosives. The reactivity of a compound towards electrophilic nitration depends on its ability to stabilize the positive charge that forms during the reaction.
Explanation:
In the given options, the most reactive compound towards electrophilic nitration is toluene (option B). Let's understand why toluene is more reactive compared to the other options.
Benzene (option A):
Benzene is a highly stable aromatic compound due to its delocalized pi-electron system. The aromaticity of benzene makes it less reactive towards electrophilic substitution reactions like nitration. The delocalization of electrons in the benzene ring helps stabilize the positive charge formed during the nitration reaction, making it less likely to proceed.
Benzoic Acid (option C):
Benzoic acid is a derivative of benzene in which a carboxylic acid group (-COOH) is attached to the aromatic ring. The presence of the electron-withdrawing carboxylic acid group reduces the electron density on the benzene ring, making it moderately reactive towards electrophilic nitration compared to benzene. However, the electron-withdrawing effect of the carboxylic acid group still hinders the reaction to some extent.
Nitrobenzene (option D):
Nitrobenzene is a compound in which a nitro group (-NO2) is already attached to the benzene ring. The presence of the nitro group strongly withdraws electron density from the ring, making nitrobenzene less reactive towards electrophilic nitration. The electron-withdrawing effect of the nitro group deactivates the ring towards further electrophilic substitution reactions.
Toluene (option B):
Toluene is a compound in which a methyl group (-CH3) is attached to the benzene ring. The methyl group is electron-donating, meaning it increases the electron density on the benzene ring. This electron-donating effect makes toluene more reactive towards electrophilic nitration compared to benzene, benzoic acid, and nitrobenzene. The increased electron density on the ring enhances the nucleophilic attack on the electrophilic nitronium ion (NO2+) formed during the nitration reaction, facilitating the reaction.
Therefore, among the given options, toluene is the most reactive compound towards electrophilic nitration due to the electron-donating effect of the methyl group.
Free Test
FREE
| Start Free Test |
Community Answer
Among the following compounds the one that is most reactive towards el...
Answer (b) is correct conclusion is toulene is more reactive towards electrophilic nitration due to presence of electron donating methyl group

|
Explore Courses for Class 11 exam
|

|
Question Description
Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer?.
Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Among the following compounds the one that is most reactive towards electrophilic nitration isa)Benzeneb)Toluenec)Benzoic Acidd)NitrobenzeneCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















