IIT JAM Exam > IIT JAM Questions > Why phenol is acidic ? give reason except res...
Start Learning for Free
Why phenol is acidic ? give reason except resonance?
Verified Answer
Why phenol is acidic ? give reason except resonance?
The acidity of phenols is due to its ability to lose hydrogen ion to form phenoxide ions. In a phenol molecule, the sp2 hybridised carbon atom of benzene ring attached directly to the hydroxyl group acts as an electron withdrawing group. This sp2 hybridized carbon atom of benzene ring attached directly to the hydroxyl group has higher electronegativity in comparison to hydroxyl group. Due to the higher Electronegativity of this carbon atom in comparison to the hydroxyl group attached, electron density decreases on oxygen atom. The decrease in electron density increases the polarity of O-H bond and results in the increase in ionization of phenols. Thus, the phenoxide ion is formed. The phenoxide ion formed is stabilized by the delocalization of negative charge due to the resonance in benzene ring. Phenoxide ion has greater stability than phenols, as in case of phenol charge separation takes place during resonance.
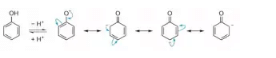
The resonance structures of phenoxide ions explain the delocalization of negative charge. In case of substituted phenols, acidity of phenols increases in the presence of electron withdrawing group. This is due to the stability of the phenoxide ion generated. The acidity of phenols further increases if these groups are attached at ortho and para positions. This is due to the fact that the negative charge in phenoxide ion is mainly delocalized at ortho and para positions of the attached benzene ring. On the other hand, the acidity of phenols decreases in presence of electron donating groups as they prohibit the formation of phenoxide ion.
 This question is part of UPSC exam. View all IIT JAM courses
This question is part of UPSC exam. View all IIT JAM courses

|
Explore Courses for IIT JAM exam
|

|
Similar IIT JAM Doubts
Why phenol is acidic ? give reason except resonance?
Question Description
Why phenol is acidic ? give reason except resonance? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about Why phenol is acidic ? give reason except resonance? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Why phenol is acidic ? give reason except resonance?.
Why phenol is acidic ? give reason except resonance? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about Why phenol is acidic ? give reason except resonance? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Why phenol is acidic ? give reason except resonance?.
Solutions for Why phenol is acidic ? give reason except resonance? in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of Why phenol is acidic ? give reason except resonance? defined & explained in the simplest way possible. Besides giving the explanation of
Why phenol is acidic ? give reason except resonance?, a detailed solution for Why phenol is acidic ? give reason except resonance? has been provided alongside types of Why phenol is acidic ? give reason except resonance? theory, EduRev gives you an
ample number of questions to practice Why phenol is acidic ? give reason except resonance? tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















