Chemistry Exam > Chemistry Questions > Which of the following expressions represent ...
Start Learning for Free
Which of the following expressions represent the first law of thermodynamics:
a. ΔU = -Q + W
b. ΔU = Q + W
c. ΔU = Q - W
d. ΔU = -Q - W
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Which of the following expressions represent the first law of thermody...
The first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes:
The first law makes use of the key concepts of internal energy, heat, and system work. It is used extensively in the discussion of heat engines. The standard unit for all these quantities would be the joule, although they are sometimes expressed in calories or BTUs.
It is typical for chemistry texts to write the first law as ΔU=Q+W. It is the same law, of course - the thermodynamic expression of the conservation of energy principle. It is just that W is defined as the work done on the system instead of work done by the system. In the context of physics, the common scenario is one of adding heat to a volume of gas and using the expansion of that gas to do work, as in the pushing down of a piston in an internal combustion engine. In the context of chemical reactions and process, it may be more common to deal with situations where work is done on the system rather than by it.
Most Upvoted Answer
Which of the following expressions represent the first law of thermody...
Explanation:
The first law of thermodynamics is also known as the law of conservation of energy. It states that energy cannot be created or destroyed, only transferred or converted from one form to another. In other words, the total energy of a system and its surroundings remains constant.
Mathematical representation:
The first law of thermodynamics can be mathematically represented using the equation:
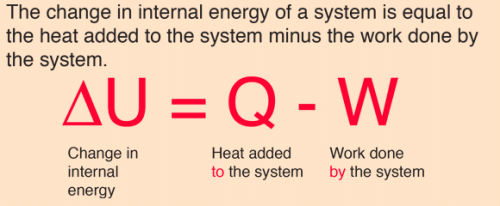
ΔU = Q - W
where ΔU is the change in internal energy of the system, Q is the heat transferred to the system, and W is the work done by the system.
Now let's break down the options given in the question:
a. ΔU = -Q + W
This equation is not correct because it implies that the internal energy of the system decreases when heat is added to it, which violates the first law of thermodynamics.
b. ΔU = Q + W
This equation is also not correct because it implies that the internal energy of the system increases when work is done on it, which again violates the first law of thermodynamics.
c. ΔU = Q - W
This equation is correct and represents the first law of thermodynamics. It states that the change in internal energy of the system is equal to the heat added to the system minus the work done by the system.
d. ΔU = -Q - W
This equation is not correct because it implies that the internal energy of the system decreases when both heat and work are added to it, which again violates the first law of thermodynamics.
Therefore, the correct answer is option 'C', which represents the first law of thermodynamics.
The first law of thermodynamics is also known as the law of conservation of energy. It states that energy cannot be created or destroyed, only transferred or converted from one form to another. In other words, the total energy of a system and its surroundings remains constant.
Mathematical representation:
The first law of thermodynamics can be mathematically represented using the equation:
ΔU = Q - W
where ΔU is the change in internal energy of the system, Q is the heat transferred to the system, and W is the work done by the system.
Now let's break down the options given in the question:
a. ΔU = -Q + W
This equation is not correct because it implies that the internal energy of the system decreases when heat is added to it, which violates the first law of thermodynamics.
b. ΔU = Q + W
This equation is also not correct because it implies that the internal energy of the system increases when work is done on it, which again violates the first law of thermodynamics.
c. ΔU = Q - W
This equation is correct and represents the first law of thermodynamics. It states that the change in internal energy of the system is equal to the heat added to the system minus the work done by the system.
d. ΔU = -Q - W
This equation is not correct because it implies that the internal energy of the system decreases when both heat and work are added to it, which again violates the first law of thermodynamics.
Therefore, the correct answer is option 'C', which represents the first law of thermodynamics.
Free Test
FREE
| Start Free Test |
Community Answer
Which of the following expressions represent the first law of thermody...
If we consider work done on the system. Then the first law of thermodynamics is,∆U= q - W. So the option B is correct

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer?
Question Description
Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer?.
Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following expressions represent the first law of thermodynamics:a. ΔU = -Q + Wb.ΔU= Q + W c.ΔU= Q - W d.ΔU = -Q - WCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
















