Class 9 Exam > Class 9 Questions > When is sublimation method for separation use...
Start Learning for Free
When is sublimation method for separation used? Explain?
Verified Answer
When is sublimation method for separation used? Explain?
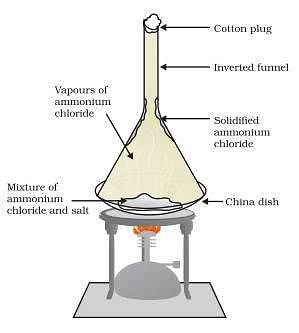
Sublimation is the property of substance in which they are converted directly from solid to gas or vice versa. Such substances are known as sublime. Some examples of solids which sublime are ammonium chloride, camphor, naphthalene and anthracene. Let us perform an activity to separate a mixture of ammonium chloride and salt.
Take a mixture of ammonium chloride and salt in a china dish cover it inverted conical transparent funnel. At the other end of the funnel put a cotton plug so that vapour could not come out. Now place china dish on a burner. As the ammonium chloride is sublime after heating it will directly converted into vapour and this vapour will again condense at the upper colder part of funnel to form solid ammonium chloride. In this way the mixture ammonium chloride and salt can be separated by the sublimation method.
Image

 This question is part of UPSC exam. View all Class 9 courses
This question is part of UPSC exam. View all Class 9 courses
Most Upvoted Answer
When is sublimation method for separation used? Explain?
Sublimation method is used when one of the substance of the mixture is a sublime substance and the other one not.Due to time the sublime substance will evaporate and the residue is the substance which does not sublime. Hope it will help you!!!!
Community Answer
When is sublimation method for separation used? Explain?
Introduction:
The sublimation method for separation is commonly used when a mixture contains a solid component that can undergo sublimation. Sublimation is the process by which a solid directly transforms into a gas without passing through the liquid phase. This method takes advantage of the different physical properties of the components in a mixture to separate them.
Principle of Sublimation:
Sublimation occurs when the vapor pressure of a solid exceeds atmospheric pressure at a given temperature. By heating the mixture to a temperature where the solid component sublimes, it can be separated from the other components.
Process of Sublimation:
The process of sublimation involves the following steps:
1. Heating: The mixture is heated to a specific temperature that allows the solid component to sublimate. This temperature is usually higher than the boiling point of the solid but lower than its decomposition temperature.
2. Conversion to Gas: As the solid component is heated, it directly transforms into a gas without passing through the liquid phase. This gas is then collected and separated from the remaining components of the mixture.
3. Cooling and Collection: The gas obtained from sublimation is cooled, causing it to condense back into a solid form. This solid is then collected and separated from any remaining gas or impurities.
Applications of Sublimation Method:
The sublimation method for separation finds applications in various fields, including:
1. Purification of Substances: Sublimation is often used to purify certain substances by removing impurities. For example, iodine can be purified through sublimation, where impurities are left behind as the iodine sublimes.
2. Separation of Components: Sublimation can be used to separate a mixture of solids when one of the components is volatile enough to undergo sublimation. This is particularly useful in cases where traditional separation methods like filtration or distillation are not applicable.
3. Production of Dry Ice: The sublimation of carbon dioxide gas is used to produce dry ice, which is widely utilized in various industries, such as food preservation, transportation, and chemical processes.
Overall, the sublimation method for separation is employed when a solid component in a mixture can undergo sublimation. It allows for the separation and purification of substances based on their different sublimation properties, providing an efficient and effective separation technique in various scientific and industrial applications.
The sublimation method for separation is commonly used when a mixture contains a solid component that can undergo sublimation. Sublimation is the process by which a solid directly transforms into a gas without passing through the liquid phase. This method takes advantage of the different physical properties of the components in a mixture to separate them.
Principle of Sublimation:
Sublimation occurs when the vapor pressure of a solid exceeds atmospheric pressure at a given temperature. By heating the mixture to a temperature where the solid component sublimes, it can be separated from the other components.
Process of Sublimation:
The process of sublimation involves the following steps:
1. Heating: The mixture is heated to a specific temperature that allows the solid component to sublimate. This temperature is usually higher than the boiling point of the solid but lower than its decomposition temperature.
2. Conversion to Gas: As the solid component is heated, it directly transforms into a gas without passing through the liquid phase. This gas is then collected and separated from the remaining components of the mixture.
3. Cooling and Collection: The gas obtained from sublimation is cooled, causing it to condense back into a solid form. This solid is then collected and separated from any remaining gas or impurities.
Applications of Sublimation Method:
The sublimation method for separation finds applications in various fields, including:
1. Purification of Substances: Sublimation is often used to purify certain substances by removing impurities. For example, iodine can be purified through sublimation, where impurities are left behind as the iodine sublimes.
2. Separation of Components: Sublimation can be used to separate a mixture of solids when one of the components is volatile enough to undergo sublimation. This is particularly useful in cases where traditional separation methods like filtration or distillation are not applicable.
3. Production of Dry Ice: The sublimation of carbon dioxide gas is used to produce dry ice, which is widely utilized in various industries, such as food preservation, transportation, and chemical processes.
Overall, the sublimation method for separation is employed when a solid component in a mixture can undergo sublimation. It allows for the separation and purification of substances based on their different sublimation properties, providing an efficient and effective separation technique in various scientific and industrial applications.
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
When is sublimation method for separation used? Explain?
Question Description
When is sublimation method for separation used? Explain? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about When is sublimation method for separation used? Explain? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When is sublimation method for separation used? Explain?.
When is sublimation method for separation used? Explain? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about When is sublimation method for separation used? Explain? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When is sublimation method for separation used? Explain?.
Solutions for When is sublimation method for separation used? Explain? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of When is sublimation method for separation used? Explain? defined & explained in the simplest way possible. Besides giving the explanation of
When is sublimation method for separation used? Explain?, a detailed solution for When is sublimation method for separation used? Explain? has been provided alongside types of When is sublimation method for separation used? Explain? theory, EduRev gives you an
ample number of questions to practice When is sublimation method for separation used? Explain? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























