JEE Exam > JEE Questions > The energy change involved in the formation o...
Start Learning for Free
The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why?
Most Upvoted Answer
The energy change involved in the formation of an iconic bond from it'...
Born-Haber Cycle
The energy change involved in the formation of ionic bond from the constituent elements can be represented by a cycle called Born-Haber Cycle.
It uses Hess’s law of thermodynamics to calculate the change in enthalpy during ionic bond formation.
Let us consider the formation of 1 mole of sodium chloride from sodium and chlorine
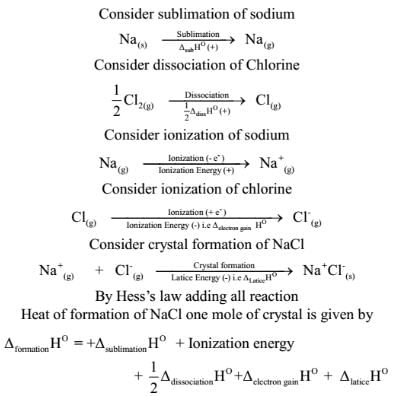

The Born-Haber Cycle can be simply stated asHeat of formation of ionic crystal = Heat of atomization + Dissociation energy+ (sum of Ionization energies)+ (sum of Electron ainities) + Lattice energy.
If energy is released (exothermic reaction), put a negative sign in front of the value of the energy; if energy
is absorbed (endothermic reaction), the value of energy should be positive.
*Note: In this general equation, the electron ainity is added. However, when plugging in a value, determine whether energy is released (exothermic reaction) or absorbed (endothermic reaction) for each electron ainity.
Community Answer
The energy change involved in the formation of an iconic bond from it'...
The energy change involved in the formation of an ionic bond from its constituent elements can be represented by: Born-Haber cycle.
Explanation:
The formation of an ionic bond involves the transfer of one or more electrons from one atom to another, resulting in the formation of ions with opposite charges. This process is accompanied by an energy change, which can be represented by the Born-Haber cycle.
Born-Haber Cycle:
The Born-Haber cycle is a series of hypothetical steps that represent the formation of an ionic compound from its constituent elements. It provides a way to calculate the lattice energy, which is the energy released when gaseous ions come together to form a solid lattice.
Key Steps in the Born-Haber Cycle:
1. Formation of gaseous atoms: The first step in the cycle is the formation of gaseous atoms from their standard states. This requires energy input, known as the sublimation energy, to overcome the forces holding the atoms in a solid lattice.
2. Ionization: The next step involves the removal of electrons from the gaseous atoms to form positive ions. This process requires energy input and is known as ionization energy.
3. Electron affinity: In this step, the gaseous atoms gain electrons to form negative ions. This process releases energy and is known as electron affinity.
4. Formation of gaseous ions: The positively and negatively charged ions combine to form gaseous ions. This step is accompanied by an energy change known as the lattice energy.
5. Formation of solid lattice: The gaseous ions come together to form a solid lattice, releasing energy. This step is also referred to as the lattice energy.
Calculation of Lattice Energy:
The lattice energy can be calculated by summing up the energy changes involved in the steps of the Born-Haber cycle. It is a measure of the strength of the ionic bond and is influenced by factors such as the charges of the ions, the distance between them, and the ionic radii.
Conclusion:
In summary, the energy change involved in the formation of an ionic bond from its constituent elements can be represented by the Born-Haber cycle. This cycle allows us to calculate the lattice energy, which is an important parameter in understanding the stability and properties of ionic compounds.
Explanation:
The formation of an ionic bond involves the transfer of one or more electrons from one atom to another, resulting in the formation of ions with opposite charges. This process is accompanied by an energy change, which can be represented by the Born-Haber cycle.
Born-Haber Cycle:
The Born-Haber cycle is a series of hypothetical steps that represent the formation of an ionic compound from its constituent elements. It provides a way to calculate the lattice energy, which is the energy released when gaseous ions come together to form a solid lattice.
Key Steps in the Born-Haber Cycle:
1. Formation of gaseous atoms: The first step in the cycle is the formation of gaseous atoms from their standard states. This requires energy input, known as the sublimation energy, to overcome the forces holding the atoms in a solid lattice.
2. Ionization: The next step involves the removal of electrons from the gaseous atoms to form positive ions. This process requires energy input and is known as ionization energy.
3. Electron affinity: In this step, the gaseous atoms gain electrons to form negative ions. This process releases energy and is known as electron affinity.
4. Formation of gaseous ions: The positively and negatively charged ions combine to form gaseous ions. This step is accompanied by an energy change known as the lattice energy.
5. Formation of solid lattice: The gaseous ions come together to form a solid lattice, releasing energy. This step is also referred to as the lattice energy.
Calculation of Lattice Energy:
The lattice energy can be calculated by summing up the energy changes involved in the steps of the Born-Haber cycle. It is a measure of the strength of the ionic bond and is influenced by factors such as the charges of the ions, the distance between them, and the ionic radii.
Conclusion:
In summary, the energy change involved in the formation of an ionic bond from its constituent elements can be represented by the Born-Haber cycle. This cycle allows us to calculate the lattice energy, which is an important parameter in understanding the stability and properties of ionic compounds.

|
Explore Courses for JEE exam
|

|
Question Description
The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why?.
The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why?.
Solutions for The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why? defined & explained in the simplest way possible. Besides giving the explanation of
The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why?, a detailed solution for The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why? has been provided alongside types of The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why? theory, EduRev gives you an
ample number of questions to practice The energy change involved in the formation of an iconic bond from it's constituent elements can be represented by A) BORN HABER cycleb) lattice energy c)internal energy d) solvation energy And please explain why? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















