Chemistry Exam > Chemistry Questions > The possible number of optical isomers in [Co...
Start Learning for Free
The possible number of optical isomers in [Co(en)2Cl2]+ are
Correct answer is '3'. Can you explain this answer?
Most Upvoted Answer
The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect ans...
[Co(en)2Cl2]Cl
Possible isomers are
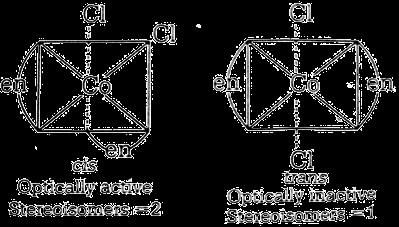
Hence,total number of stereoisomers = 2+1 = 3
Free Test
FREE
| Start Free Test |
Community Answer
The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect ans...
Introduction:
Optical isomers are stereoisomers that have the same molecular formula and connectivity but differ in their spatial arrangement. [Co(en)2Cl2] is a coordination compound in which the central cobalt atom is coordinated with two ethylenediamine ligands and two chloride ions.
Determination of optical isomers:
To determine the number of optical isomers, we need to consider the presence of chiral centers in the molecule. A chiral center is an atom in a molecule that is bonded to four different groups. In [Co(en)2Cl2], the two nitrogen atoms in the ethylenediamine ligands are chiral centers.
Calculation of possible isomers:
The possible number of optical isomers can be calculated using the formula 2^n, where n is the number of chiral centers. In this case, there are two chiral centers, so the total number of possible isomers is 2^2 = 4.
However, not all of these isomers are actually possible due to the presence of a plane of symmetry in the molecule. A plane of symmetry is an imaginary plane that can be drawn through a molecule so that one half of the molecule is the mirror image of the other half.
Elimination of impossible isomers:
In [Co(en)2Cl2], there is a plane of symmetry that passes through the central cobalt atom and bisects the two ethylenediamine ligands. This means that the two possible mirror images of the molecule are superimposable, and therefore not true optical isomers. The remaining two isomers are:
- Enantiomer 1: The two ethylenediamine ligands are arranged in a clockwise manner around the cobalt atom.
- Enantiomer 2: The two ethylenediamine ligands are arranged in a counterclockwise manner around the cobalt atom.
These two isomers are true optical isomers, as they are non-superimposable mirror images of each other. Therefore, the possible number of optical isomers in [Co(en)2Cl2] is 2. However, we must also consider the fact that the chloride ions can occupy two different positions in the molecule, resulting in a total of 2 x 2 = 4 possible isomers. Out of these four, only three are actually possible due to the presence of the plane of symmetry. Thus, the correct answer is 3.
Optical isomers are stereoisomers that have the same molecular formula and connectivity but differ in their spatial arrangement. [Co(en)2Cl2] is a coordination compound in which the central cobalt atom is coordinated with two ethylenediamine ligands and two chloride ions.
Determination of optical isomers:
To determine the number of optical isomers, we need to consider the presence of chiral centers in the molecule. A chiral center is an atom in a molecule that is bonded to four different groups. In [Co(en)2Cl2], the two nitrogen atoms in the ethylenediamine ligands are chiral centers.
Calculation of possible isomers:
The possible number of optical isomers can be calculated using the formula 2^n, where n is the number of chiral centers. In this case, there are two chiral centers, so the total number of possible isomers is 2^2 = 4.
However, not all of these isomers are actually possible due to the presence of a plane of symmetry in the molecule. A plane of symmetry is an imaginary plane that can be drawn through a molecule so that one half of the molecule is the mirror image of the other half.
Elimination of impossible isomers:
In [Co(en)2Cl2], there is a plane of symmetry that passes through the central cobalt atom and bisects the two ethylenediamine ligands. This means that the two possible mirror images of the molecule are superimposable, and therefore not true optical isomers. The remaining two isomers are:
- Enantiomer 1: The two ethylenediamine ligands are arranged in a clockwise manner around the cobalt atom.
- Enantiomer 2: The two ethylenediamine ligands are arranged in a counterclockwise manner around the cobalt atom.
These two isomers are true optical isomers, as they are non-superimposable mirror images of each other. Therefore, the possible number of optical isomers in [Co(en)2Cl2] is 2. However, we must also consider the fact that the chloride ions can occupy two different positions in the molecule, resulting in a total of 2 x 2 = 4 possible isomers. Out of these four, only three are actually possible due to the presence of the plane of symmetry. Thus, the correct answer is 3.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Question Description
The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer?.
The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer?.
Solutions for The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer?, a detailed solution for The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer? has been provided alongside types of The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The possible number of optical isomers in [Co(en)2Cl2]+ areCorrect answer is '3'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















