JEE Exam > JEE Questions > According to Molecular Orbital Theory, (JEE A...
Start Learning for Free
According to Molecular Orbital Theory, (JEE Adv. 2016)
- a)C22- is expected to be diamagnetic
- b)O22+ is expected to have a longer bond length than O2
- c)N+2 an d N2- have th e same bond or der
- d)He+2 has the same ener gy as two isolated He atoms
Correct answer is option 'A,C'. Can you explain this answer?
Most Upvoted Answer
According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expect...
(A) The molecular orbital energy configuration of C22– is
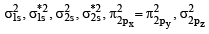
In the MO of C22– there is no unpaired electron hence it is diamagnetic
(B) Bond order of O22+ is 3 and O2 is 2 therefore bond length of O2 is greater than O22+
(C) The molecular orbital energy configuration of N+2 is
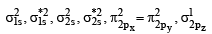
Bond order of 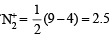
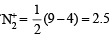
The molecular orbital energy configuration of N–2 is
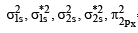
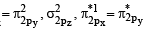
Bond order of 
(D) He+2 has less energy in comparison to two isolated He atoms because some energy is released during the formation of He+2 from 2 He atoms.
Free Test
| FREE | Start Free Test |
Community Answer
According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expect...
Molecular Orbital Theory and its Applications:
Molecular Orbital Theory (MOT) is a theoretical approach used to describe the nature of chemical bonding in molecules. MOT is based on the concept of overlapping atomic orbitals to form molecular orbitals (MOs).
(a) C22- is expected to be diamagnetic:
- Carbon has six valence electrons (2s2 2p2) and when two carbon atoms combine, they form four molecular orbitals (MOs).
- The two lowest-energy MOs are bonding MOs and the two highest-energy MOs are antibonding MOs.
- When C22- is formed by adding two electrons to the antibonding MO, the total number of electrons becomes 12, which is an even number.
- According to Hund's rule, electrons fill up MOs singly with parallel spins before pairing up. Since all the electrons are paired up in C22-, it is diamagnetic.
(b) O22 is expected to have a longer bond length than O2:
- Oxygen has eight valence electrons (2s2 2p4) and when two oxygen atoms combine, they form eight MOs.
- The two lowest-energy MOs are bonding MOs and the two highest-energy MOs are antibonding MOs.
- In O2, there are two unpaired electrons in the antibonding MO, which results in a shorter bond length.
- However, in O22, the two electrons in the antibonding MO pair up, which results in a longer bond length.
(c) N2 and N2- have the same bond order:
- Nitrogen has seven valence electrons (2s2 2p3) and when two nitrogen atoms combine, they form ten MOs.
- The two lowest-energy MOs are bonding MOs and the two highest-energy MOs are antibonding MOs.
- The bond order is given by the difference between the number of electrons in the bonding MOs and the number of electrons in the antibonding MOs divided by 2.
- For N2, the bond order is (5-2)/2 = 1.5 and for N2-, the bond order is (5-3)/2 = 1.
- Therefore, N2 and N2- have the same bond order.
(d) He2 has the same energy as two isolated He atoms:
- Helium has two valence electrons (1s2) and when two helium atoms combine, they form four MOs.
- The two lowest-energy MOs are bonding MOs and the two highest-energy MOs are antibonding MOs.
- Since there are no unpaired electrons in He2, the energy of He2 is the same as two isolated He atoms.
Molecular Orbital Theory (MOT) is a theoretical approach used to describe the nature of chemical bonding in molecules. MOT is based on the concept of overlapping atomic orbitals to form molecular orbitals (MOs).
(a) C22- is expected to be diamagnetic:
- Carbon has six valence electrons (2s2 2p2) and when two carbon atoms combine, they form four molecular orbitals (MOs).
- The two lowest-energy MOs are bonding MOs and the two highest-energy MOs are antibonding MOs.
- When C22- is formed by adding two electrons to the antibonding MO, the total number of electrons becomes 12, which is an even number.
- According to Hund's rule, electrons fill up MOs singly with parallel spins before pairing up. Since all the electrons are paired up in C22-, it is diamagnetic.
(b) O22 is expected to have a longer bond length than O2:
- Oxygen has eight valence electrons (2s2 2p4) and when two oxygen atoms combine, they form eight MOs.
- The two lowest-energy MOs are bonding MOs and the two highest-energy MOs are antibonding MOs.
- In O2, there are two unpaired electrons in the antibonding MO, which results in a shorter bond length.
- However, in O22, the two electrons in the antibonding MO pair up, which results in a longer bond length.
(c) N2 and N2- have the same bond order:
- Nitrogen has seven valence electrons (2s2 2p3) and when two nitrogen atoms combine, they form ten MOs.
- The two lowest-energy MOs are bonding MOs and the two highest-energy MOs are antibonding MOs.
- The bond order is given by the difference between the number of electrons in the bonding MOs and the number of electrons in the antibonding MOs divided by 2.
- For N2, the bond order is (5-2)/2 = 1.5 and for N2-, the bond order is (5-3)/2 = 1.
- Therefore, N2 and N2- have the same bond order.
(d) He2 has the same energy as two isolated He atoms:
- Helium has two valence electrons (1s2) and when two helium atoms combine, they form four MOs.
- The two lowest-energy MOs are bonding MOs and the two highest-energy MOs are antibonding MOs.
- Since there are no unpaired electrons in He2, the energy of He2 is the same as two isolated He atoms.

|
Explore Courses for JEE exam
|

|
Question Description
According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer?.
According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer?.
Solutions for According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer?, a detailed solution for According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer? has been provided alongside types of According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice According to Molecular Orbital Theory, (JEE Adv. 2016)a)C22- is expected to be diamagneticb)O22+ is expected to have a longer bond length than O2c)N+2 an d N2- have th e same bond or derd)He+2 has the same ener gy as two isolated He atomsCorrect answer is option 'A,C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























