Class 12 Exam > Class 12 Questions > The antiseptic properties of iodoform is due ...
Start Learning for Free
The antiseptic properties of iodoform is due to which of the following:
- a)Liberation of iodine
- b)Smell of iodoform
- c)Colour of iodoform
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The antiseptic properties of iodoform is due to which of the following...
The antiseptic properties of iodoform is due to liberation of iodine.
View all questions of this test
Most Upvoted Answer
The antiseptic properties of iodoform is due to which of the following...
Iodoform liberates I2, when it comes in contact with skin Antiseptic property of iodine is due to the liberation of I2, not because of iodoform itself.
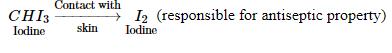
Free Test
FREE
| Start Free Test |
Community Answer
The antiseptic properties of iodoform is due to which of the following...
Antiseptic properties of iodoform are due to the liberation of iodine.
Explanation:
Iodoform, also known as triiodomethane (CHI3), is a yellow crystalline solid with a strong antiseptic property. It has been used for many years as an effective antiseptic in medicine. The antiseptic property of iodoform is primarily due to the liberation of iodine when it comes into contact with body fluids or tissues. Let's understand this in detail:
1. Formation of iodoform:
Iodoform is formed by the reaction of ethanol (or any alcohol) with iodine and sodium hydroxide. This reaction is known as the iodoform test and is used to detect the presence of methyl ketones in organic compounds.
2. Liberation of iodine:
When iodoform comes into contact with body fluids or tissues, it undergoes a slow decomposition. This decomposition results in the liberation of iodine. The liberated iodine acts as an antiseptic agent, killing or inhibiting the growth of microorganisms such as bacteria, fungi, and viruses.
3. Mechanism of action:
Iodine, as a halogen, has strong oxidizing properties. It can penetrate the cell wall of microorganisms and disrupt their metabolic processes, leading to their death. Iodine also inhibits the synthesis of proteins and nucleic acids in microorganisms, further contributing to their destruction.
4. Broad-spectrum activity:
The antiseptic property of iodoform is effective against a wide range of microorganisms, including bacteria (both Gram-positive and Gram-negative), fungi, and viruses. This broad-spectrum activity makes iodoform a valuable antiseptic agent in various medical applications.
5. Other factors:
The smell and color of iodoform are not directly responsible for its antiseptic properties. The characteristic smell of iodoform is due to the presence of triiodomethane molecules, which have a distinct odor. The yellow color of iodoform is a result of its molecular structure and does not contribute to its antiseptic activity.
In conclusion, the antiseptic properties of iodoform are primarily due to the liberation of iodine when it comes into contact with body fluids or tissues. The liberated iodine acts as a potent antimicrobial agent, killing or inhibiting the growth of microorganisms.
Explanation:
Iodoform, also known as triiodomethane (CHI3), is a yellow crystalline solid with a strong antiseptic property. It has been used for many years as an effective antiseptic in medicine. The antiseptic property of iodoform is primarily due to the liberation of iodine when it comes into contact with body fluids or tissues. Let's understand this in detail:
1. Formation of iodoform:
Iodoform is formed by the reaction of ethanol (or any alcohol) with iodine and sodium hydroxide. This reaction is known as the iodoform test and is used to detect the presence of methyl ketones in organic compounds.
2. Liberation of iodine:
When iodoform comes into contact with body fluids or tissues, it undergoes a slow decomposition. This decomposition results in the liberation of iodine. The liberated iodine acts as an antiseptic agent, killing or inhibiting the growth of microorganisms such as bacteria, fungi, and viruses.
3. Mechanism of action:
Iodine, as a halogen, has strong oxidizing properties. It can penetrate the cell wall of microorganisms and disrupt their metabolic processes, leading to their death. Iodine also inhibits the synthesis of proteins and nucleic acids in microorganisms, further contributing to their destruction.
4. Broad-spectrum activity:
The antiseptic property of iodoform is effective against a wide range of microorganisms, including bacteria (both Gram-positive and Gram-negative), fungi, and viruses. This broad-spectrum activity makes iodoform a valuable antiseptic agent in various medical applications.
5. Other factors:
The smell and color of iodoform are not directly responsible for its antiseptic properties. The characteristic smell of iodoform is due to the presence of triiodomethane molecules, which have a distinct odor. The yellow color of iodoform is a result of its molecular structure and does not contribute to its antiseptic activity.
In conclusion, the antiseptic properties of iodoform are primarily due to the liberation of iodine when it comes into contact with body fluids or tissues. The liberated iodine acts as a potent antimicrobial agent, killing or inhibiting the growth of microorganisms.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer?
Question Description
The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer?.
The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer?.
Solutions for The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The antiseptic properties of iodoform is due to which of the following:a)Liberation of iodineb)Smell of iodoformc)Colour of iodoformd)None of the aboveCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















