Chemistry Exam > Chemistry Questions > Which have lowest bond angle?a)CH4b)NH3c)PH3d...
Start Learning for Free
Which have lowest bond angle?
- a)CH4
- b)NH3
- c)PH3
- d)H20
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is opt...
Most Upvoted Answer
Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is opt...
**Explanation:**
**Bond angle** is the angle formed between two adjacent bonds in a molecule. The bond angle is influenced by the repulsion between the electron pairs around the central atom. The greater the repulsion, the larger the bond angle.
In this question, we need to determine which molecule has the lowest bond angle among CH4, NH3, PH3, and H2O.
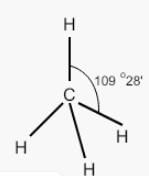
1. **CH4 (Methane):** Methane has a tetrahedral molecular geometry with four hydrogen atoms surrounding the central carbon atom. Each hydrogen atom is bonded to the carbon atom by a single covalent bond. The bond angle in methane is 109.5°.
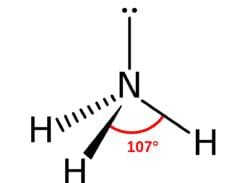
2. **NH3 (Ammonia):** Ammonia has a trigonal pyramidal molecular geometry with three hydrogen atoms and one lone pair of electrons surrounding the central nitrogen atom. The bond angle in ammonia is 107°.
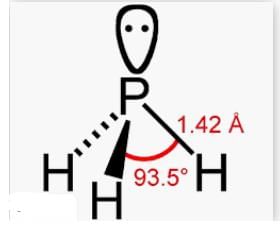
3. **PH3 (Phosphine):** Phosphine has a trigonal pyramidal molecular geometry similar to ammonia, with three hydrogen atoms and one lone pair of electrons surrounding the central phosphorus atom. However, the bond angle in phosphine is larger than in ammonia. The bond angle in phosphine is approximately 93°.
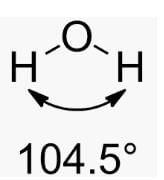
4. **H2O (Water):** Water has a bent or V-shaped molecular geometry with two hydrogen atoms and two lone pairs of electrons surrounding the central oxygen atom. The bond angle in water is approximately 104.5°.
Based on the given options, we can see that the bond angle in PH3 (93°) is smaller than the bond angles in all the other options. Therefore, the correct answer is option **C) PH3**.
It is important to note that the bond angles mentioned here are approximate values and can vary slightly due to factors such as lone pair repulsion and bond length.
**Bond angle** is the angle formed between two adjacent bonds in a molecule. The bond angle is influenced by the repulsion between the electron pairs around the central atom. The greater the repulsion, the larger the bond angle.
In this question, we need to determine which molecule has the lowest bond angle among CH4, NH3, PH3, and H2O.
1. **CH4 (Methane):** Methane has a tetrahedral molecular geometry with four hydrogen atoms surrounding the central carbon atom. Each hydrogen atom is bonded to the carbon atom by a single covalent bond. The bond angle in methane is 109.5°.
2. **NH3 (Ammonia):** Ammonia has a trigonal pyramidal molecular geometry with three hydrogen atoms and one lone pair of electrons surrounding the central nitrogen atom. The bond angle in ammonia is 107°.
3. **PH3 (Phosphine):** Phosphine has a trigonal pyramidal molecular geometry similar to ammonia, with three hydrogen atoms and one lone pair of electrons surrounding the central phosphorus atom. However, the bond angle in phosphine is larger than in ammonia. The bond angle in phosphine is approximately 93°.
4. **H2O (Water):** Water has a bent or V-shaped molecular geometry with two hydrogen atoms and two lone pairs of electrons surrounding the central oxygen atom. The bond angle in water is approximately 104.5°.
Based on the given options, we can see that the bond angle in PH3 (93°) is smaller than the bond angles in all the other options. Therefore, the correct answer is option **C) PH3**.
It is important to note that the bond angles mentioned here are approximate values and can vary slightly due to factors such as lone pair repulsion and bond length.
Free Test
FREE
| Start Free Test |
Community Answer
Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is opt...
PH3 bcs it follows drago rule and hence there would be no hybridisation and hence simple p orbital which is at 90 degree. Therefore bond angle will be almost equal to 90 degree.

|
Explore Courses for Chemistry exam
|

|
Question Description
Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer?.
Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer?.
Solutions for Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which have lowest bond angle?a)CH4b)NH3c)PH3d)H20Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.