Class 12 Exam > Class 12 Questions > Water is added to the solution M such that th...
Start Learning for Free
Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K?
Verified Answer
Water is added to the solution M such that the mole fraction of water ...
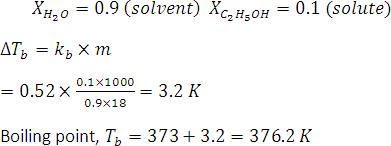
 This question is part of UPSC exam. View all Class 12 courses
This question is part of UPSC exam. View all Class 12 courses
Most Upvoted Answer
Water is added to the solution M such that the mole fraction of water ...
Introduction:
In this scenario, water is added to a solution M to increase the mole fraction of water in the solution to 0.9. We need to determine the boiling point of this solution.
Explanation:
To calculate the boiling point of the solution, we can use the concept of boiling point elevation. According to Raoult's law, the boiling point of a solution is higher than that of the pure solvent due to the presence of solute particles.
Boiling Point Elevation:
The boiling point elevation (∆Tb) can be calculated using the formula:
∆Tb = Kb * m * i
where:
- ∆Tb is the boiling point elevation
- Kb is the molal boiling point elevation constant (a characteristic of the solvent)
- m is the molality of the solute (moles of solute per kg of solvent)
- i is the van't Hoff factor (the number of particles into which a compound dissociates in a solution)
Determination of ∆Tb:
Since we are adding water to the solution M, the solvent remains water. Therefore, we need to determine the molality of the solute in the solution.
The mole fraction of water (Xwater) in the solution is given as 0.9. The mole fraction can be calculated using the formula:
Xwater = moles of water / (moles of water + moles of solute)
Since the mole fraction of water is 0.9, the mole fraction of the solute (Xsolute) can be calculated as:
Xsolute = 1 - Xwater = 1 - 0.9 = 0.1
This means that the mole fraction of the solute is 0.1.
Determination of ∆Tb:
To calculate the boiling point elevation, we need to know the molal boiling point elevation constant (Kb). Since the solvent is water, we can use the Kb value for water, which is approximately 0.512 °C/m.
We also need to determine the molality of the solute (m). The molality can be calculated using the formula:
m = moles of solute / mass of solvent (in kg)
Since the mole fraction of the solute is 0.1, the mole fraction of water is 0.9, and the total moles of solute and water should add up to 1, we can assume that the mass of the solvent is 1 kg.
Therefore, the molality of the solute is:
m = moles of solute / 1 kg
We can substitute these values into the boiling point elevation formula:
∆Tb = 0.512 °C/m * moles of solute / 1 kg * i
Since the solute is not specified in the problem, we cannot determine the van't Hoff factor (i) without additional information. However, for most solutes, i is equal to 1. Therefore, we can assume i = 1.
Conclusion:
Based on the given information, we can calculate the boiling point elevation (∆Tb) of the solution using the formula mentioned above. However, since the van't Hoff factor (i) is not provided, we cannot determine the exact value of ∆Tb.
Therefore, we
In this scenario, water is added to a solution M to increase the mole fraction of water in the solution to 0.9. We need to determine the boiling point of this solution.
Explanation:
To calculate the boiling point of the solution, we can use the concept of boiling point elevation. According to Raoult's law, the boiling point of a solution is higher than that of the pure solvent due to the presence of solute particles.
Boiling Point Elevation:
The boiling point elevation (∆Tb) can be calculated using the formula:
∆Tb = Kb * m * i
where:
- ∆Tb is the boiling point elevation
- Kb is the molal boiling point elevation constant (a characteristic of the solvent)
- m is the molality of the solute (moles of solute per kg of solvent)
- i is the van't Hoff factor (the number of particles into which a compound dissociates in a solution)
Determination of ∆Tb:
Since we are adding water to the solution M, the solvent remains water. Therefore, we need to determine the molality of the solute in the solution.
The mole fraction of water (Xwater) in the solution is given as 0.9. The mole fraction can be calculated using the formula:
Xwater = moles of water / (moles of water + moles of solute)
Since the mole fraction of water is 0.9, the mole fraction of the solute (Xsolute) can be calculated as:
Xsolute = 1 - Xwater = 1 - 0.9 = 0.1
This means that the mole fraction of the solute is 0.1.
Determination of ∆Tb:
To calculate the boiling point elevation, we need to know the molal boiling point elevation constant (Kb). Since the solvent is water, we can use the Kb value for water, which is approximately 0.512 °C/m.
We also need to determine the molality of the solute (m). The molality can be calculated using the formula:
m = moles of solute / mass of solvent (in kg)
Since the mole fraction of the solute is 0.1, the mole fraction of water is 0.9, and the total moles of solute and water should add up to 1, we can assume that the mass of the solvent is 1 kg.
Therefore, the molality of the solute is:
m = moles of solute / 1 kg
We can substitute these values into the boiling point elevation formula:
∆Tb = 0.512 °C/m * moles of solute / 1 kg * i
Since the solute is not specified in the problem, we cannot determine the van't Hoff factor (i) without additional information. However, for most solutes, i is equal to 1. Therefore, we can assume i = 1.
Conclusion:
Based on the given information, we can calculate the boiling point elevation (∆Tb) of the solution using the formula mentioned above. However, since the van't Hoff factor (i) is not provided, we cannot determine the exact value of ∆Tb.
Therefore, we

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K?
Question Description
Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K?.
Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K?.
Solutions for Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K? defined & explained in the simplest way possible. Besides giving the explanation of
Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K?, a detailed solution for Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K? has been provided alongside types of Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K? theory, EduRev gives you an
ample number of questions to practice Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is : ans is 376.2K? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















