Class 11 Exam > Class 11 Questions > How many different stereoisomers exist for 1-...
Start Learning for Free
How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?
a)2
b)4
c)5
d)7
Correct answer is option 'B'. Can you explain this answer?
Most Upvoted Answer
How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobu...
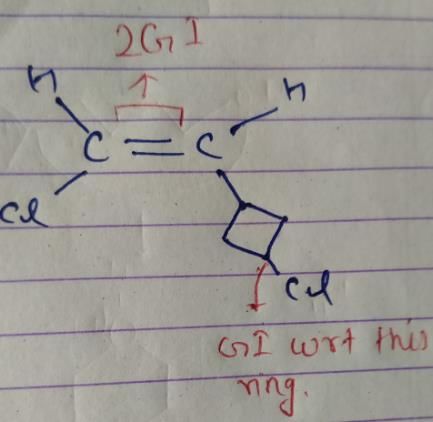
So, there are 4 isomers of given compound.
Free Test
FREE
| Start Free Test |
Community Answer
How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobu...
Stereoisomerism in organic chemistry arises due to the presence of stereocenters or double bonds with restricted rotation. Stereoisomers have the same molecular formula and connectivity, but differ in the arrangement of their atoms in space.
Given compound: 1-chloro-2-(3-chlorocyclobutane) ethene
Step 1: Identify stereocenters or double bonds with restricted rotation
The given compound has a double bond between carbon 1 and carbon 2. The carbon atoms in the cyclobutane ring are also stereocenters, as they have four different substituents attached to them.
Step 2: Determine the number of stereoisomers
The number of stereoisomers can be determined using the following formula:
Number of stereoisomers = 2^n, where n is the number of stereocenters or double bonds with restricted rotation.
In the given compound, there are two stereocenters in the cyclobutane ring and a double bond between carbon 1 and carbon 2. Therefore, the number of stereoisomers = 2^2 x 2^1 = 4 x 2 = 8.
However, some of these stereoisomers may be identical due to symmetry. In this case, the cyclobutane ring has a plane of symmetry, which means that some of the stereoisomers are identical to their mirror images. Therefore, the actual number of different stereoisomers is less than 8.
Step 3: Identify identical stereoisomers
The two stereocenters in the cyclobutane ring can have two different configurations: cis or trans. However, the cis and trans stereoisomers are mirror images of each other and are therefore identical. This reduces the number of stereoisomers to 4.
Step 4: Final answer
Therefore, the number of different stereoisomers for 1-chloro-2-(3-chlorocyclobutane) ethene is 4. The correct answer is option B.
Given compound: 1-chloro-2-(3-chlorocyclobutane) ethene
Step 1: Identify stereocenters or double bonds with restricted rotation
The given compound has a double bond between carbon 1 and carbon 2. The carbon atoms in the cyclobutane ring are also stereocenters, as they have four different substituents attached to them.
Step 2: Determine the number of stereoisomers
The number of stereoisomers can be determined using the following formula:
Number of stereoisomers = 2^n, where n is the number of stereocenters or double bonds with restricted rotation.
In the given compound, there are two stereocenters in the cyclobutane ring and a double bond between carbon 1 and carbon 2. Therefore, the number of stereoisomers = 2^2 x 2^1 = 4 x 2 = 8.
However, some of these stereoisomers may be identical due to symmetry. In this case, the cyclobutane ring has a plane of symmetry, which means that some of the stereoisomers are identical to their mirror images. Therefore, the actual number of different stereoisomers is less than 8.
Step 3: Identify identical stereoisomers
The two stereocenters in the cyclobutane ring can have two different configurations: cis or trans. However, the cis and trans stereoisomers are mirror images of each other and are therefore identical. This reduces the number of stereoisomers to 4.
Step 4: Final answer
Therefore, the number of different stereoisomers for 1-chloro-2-(3-chlorocyclobutane) ethene is 4. The correct answer is option B.

|
Explore Courses for Class 11 exam
|

|
Question Description
How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer?.
How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer?.
Solutions for How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer?, a detailed solution for How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice How many different stereoisomers exist for 1-chloro-2-(3-chlorocyclobutane) ethene?a)2b)4c)5d)7Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.