Chemistry Exam > Chemistry Questions > NMR spectroscopy can be used to assay for dru...
Start Learning for Free
NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of 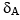 while that of the free from resonated at
while that of the free from resonated at 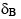 for the same proton (see figure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:
for the same proton (see figure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:
- a)The NMR line width of the observed transition(s) will be different from the free form of the drug
- b)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.
- c)We will see a single resonance at a posit ion
f which in between
and
- d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer?
Question Description
NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer?.
NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer?.
Solutions for NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer?, a detailed solution for NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice NMR spectroscopy can be used to assay for drug binding to certain protein targets. One of the primary objective of any binding assay is the quantification of the free and the bound forms of a drug molecule at a certain concentration of the protein target. Let us assume that the fully bound drug exhibits a1H chemical shift of while that of the free from resonated at for the same proton (seefigure above). If the exchange timescale (i.e. proportional to kOFF as kON is diffusion limited) between the free form of the drug and its bound form is in microseconds, which of the following statements cannot be true assuming that the drug is only 50% bound with its protein target:a)The NMR line width of the observed transition(s) will be different from the free form of the drugb)There will be two resonance obtained in the NMR spectrum of the Protein-Drug complex: One for the free form while other for the bound form.c)We will see a single resonance at a posit ion f which in between and d)Varying the concentration of the drug molecule while observing the NMR signatures will provide an estimate of the binding constant.Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















