Chemistry Exam > Chemistry Questions > During oxygen transport by hemerythrin, oxyge...
Start Learning for Free
During oxygen transport by hemerythrin, oxygen is bound as:
- a)O2– to one Fe(III) only
- b)HO2– to one Fe(III) only
- c)O22– to one Fe(II) and one Fe(III)
- d)O22– to two Fe(II)
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
During oxygen transport by hemerythrin, oxygen is bound as:a)O2–...
The mechanism of dioxygen binding is unusual. Most O2 carriers operate via formation of dioxygen complexes, but hemerythrin holds the O2 as a hydroperoxide (HO2, or -OOH−). The site that binds O2 consists of a pair of iron centres. The iron atoms are bound to the protein through the carboxylate side chains of a glutamate and aspartates as well as through five histidine residues. Hemerythrin and myohemerythrin are often described according to oxidation and ligation states of the iron center:
Fe2+—OH—Fe2+deoxy (reduced)
Fe2+—OH—Fe3+semi-met
Fe3+—O—Fe3+—OOH−oxy (oxidized)
Fe3+—OH—Fe3+— (any other ligand)met (oxidized)
The uptake of O2 by hemerythrin is accompanied by two-electron oxidation of the diferrous centre to produce a hydroperoxide (OOH−) complex. The binding of O2 is roughly described in this diagram:
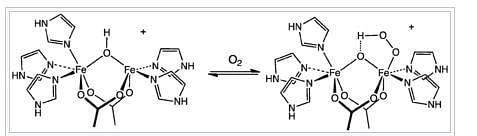
Most Upvoted Answer
During oxygen transport by hemerythrin, oxygen is bound as:a)O2–...
Oxygen transport by hemerythrin
Hemerythrin is a respiratory protein found in some marine invertebrates. It is involved in the transport and storage of oxygen, similar to hemoglobin in vertebrates. Hemerythrin contains iron ions that bind to oxygen molecules, allowing for efficient oxygen transport.
Binding of oxygen to hemerythrin
The binding of oxygen to hemerythrin occurs through the coordination of oxygen molecules to the iron ions present in the protein. Each hemerythrin molecule contains two iron ions, typically in the +2 and +3 oxidation states. Oxygen can bind to either the +2 or +3 iron ion, resulting in different forms of oxygen-bound hemerythrin.
Options for oxygen binding
a) O2 to one Fe(III) only
- This option suggests that oxygen molecules bind to the +3 iron ion only. However, this is not the case as hemerythrin can bind oxygen to both the +2 and +3 iron ions.
b) HO2 to one Fe(III) only
- This option suggests that oxygen binds as HO2 to the +3 iron ion. HO2 refers to a hydroperoxy group (-OOH) attached to the iron ion. This is the correct option as it accurately describes the binding of oxygen to hemerythrin.
c) O22 to one Fe(II) and one Fe(III)
- This option suggests that oxygen molecules bind to both the +2 and +3 iron ions. However, oxygen binding typically occurs to either the +2 or +3 iron ion, not both simultaneously.
d) O22 to two Fe(II)
- This option suggests that oxygen molecules bind to two +2 iron ions. However, oxygen binding typically occurs to either the +2 or +3 iron ion, not two +2 ions.
Conclusion
The correct answer is option 'b' - HO2 binds to one Fe(III) only. This accurately describes the binding of oxygen to hemerythrin, where oxygen molecules coordinate to the +3 iron ion in the form of a hydroperoxy group (-OOH). This binding allows for efficient oxygen transport and release in the organism.
Hemerythrin is a respiratory protein found in some marine invertebrates. It is involved in the transport and storage of oxygen, similar to hemoglobin in vertebrates. Hemerythrin contains iron ions that bind to oxygen molecules, allowing for efficient oxygen transport.
Binding of oxygen to hemerythrin
The binding of oxygen to hemerythrin occurs through the coordination of oxygen molecules to the iron ions present in the protein. Each hemerythrin molecule contains two iron ions, typically in the +2 and +3 oxidation states. Oxygen can bind to either the +2 or +3 iron ion, resulting in different forms of oxygen-bound hemerythrin.
Options for oxygen binding
a) O2 to one Fe(III) only
- This option suggests that oxygen molecules bind to the +3 iron ion only. However, this is not the case as hemerythrin can bind oxygen to both the +2 and +3 iron ions.
b) HO2 to one Fe(III) only
- This option suggests that oxygen binds as HO2 to the +3 iron ion. HO2 refers to a hydroperoxy group (-OOH) attached to the iron ion. This is the correct option as it accurately describes the binding of oxygen to hemerythrin.
c) O22 to one Fe(II) and one Fe(III)
- This option suggests that oxygen molecules bind to both the +2 and +3 iron ions. However, oxygen binding typically occurs to either the +2 or +3 iron ion, not both simultaneously.
d) O22 to two Fe(II)
- This option suggests that oxygen molecules bind to two +2 iron ions. However, oxygen binding typically occurs to either the +2 or +3 iron ion, not two +2 ions.
Conclusion
The correct answer is option 'b' - HO2 binds to one Fe(III) only. This accurately describes the binding of oxygen to hemerythrin, where oxygen molecules coordinate to the +3 iron ion in the form of a hydroperoxy group (-OOH). This binding allows for efficient oxygen transport and release in the organism.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer?
Question Description
During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer?.
During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer?.
Solutions for During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer?, a detailed solution for During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice During oxygen transport by hemerythrin, oxygen is bound as:a)O2– to one Fe(III) onlyb)HO2– to one Fe(III) onlyc)O22– to one Fe(II) and one Fe(III)d)O22– to two Fe(II)Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.













