Chemistry Exam > Chemistry Questions > In a typical Conductometric titration of a st...
Start Learning for Free
In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:
- a)
- b)
- c)
- d)
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
In a typical Conductometric titration of a strong acid with a weak bas...
Here consider a strong acid as HCL and weak base as Ammonium hydroxide that is NH4OH. Suppose the acid has high concentration of H positive ions due to which it show high conductance, when a weak base is added to it, the cation of weak base combines with Cl Negative ion to form ammonium chloride precipitate thus decreasing the conductivity by utilizing H positive ion to form water molecule due to this decrease in conductivity the graph shows on negative fall in conductance after all the edge positive and Cl negative ions are combine end point is reached where no free ions are possible for conduction after this when the basis added to form NH4 positive and was OH negative since it is a weak base it has low conductivity and the graph increases very slowly towards conduction minute changes in increasing conduction can be seen when a weak bases are added
plot a) show high conductivity which is not possible in weak bases
plot c) and d) involve 3 mixture components which is not the case
The perfect diagram would look like this and B is the closest.
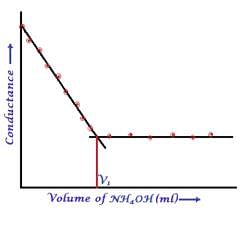
plot c) and d) involve 3 mixture components which is not the case
The perfect diagram would look like this and B is the closest.

Most Upvoted Answer
In a typical Conductometric titration of a strong acid with a weak bas...
Correct answer is b
Here consider a strong acid as HCL and weak base as Ammonium hydroxide that is NH 4 0h suppose acid is present acid has high concentration of h positive ions due to which it show high conductance when a weak base is added to it the cation of weak base combines with CL Negative Ion to form ammonium chloride precipitate does decreasing the conductivity by utilising H positive ion to form water molecule due to this decrease in conductivity the graph shows on negative fall in conductance after all the edge positive and CL negative ions are combine end point is reached where no free ions are possible for conduction after this when the basis added to form NH4 positive and was o h negative since it is a weak base it has low conductivity and the graph increases very slowly towards conduction minute changes in increasing conduction can be seen when a weak bases are added
plot a) show high conductivity which is not possible in weak bases
plot c) and d) involve 3 mixture components which is not the case
Here consider a strong acid as HCL and weak base as Ammonium hydroxide that is NH 4 0h suppose acid is present acid has high concentration of h positive ions due to which it show high conductance when a weak base is added to it the cation of weak base combines with CL Negative Ion to form ammonium chloride precipitate does decreasing the conductivity by utilising H positive ion to form water molecule due to this decrease in conductivity the graph shows on negative fall in conductance after all the edge positive and CL negative ions are combine end point is reached where no free ions are possible for conduction after this when the basis added to form NH4 positive and was o h negative since it is a weak base it has low conductivity and the graph increases very slowly towards conduction minute changes in increasing conduction can be seen when a weak bases are added
plot a) show high conductivity which is not possible in weak bases
plot c) and d) involve 3 mixture components which is not the case
Free Test
FREE
| Start Free Test |
Community Answer
In a typical Conductometric titration of a strong acid with a weak bas...
Here consider a strong acid as HCL and weak base as Ammonium hydroxide that is NH4OH. Suppose the acid has high concentration of H positive ions due to which it show high conductance, when a weak base is added to it, the cation of weak base combines with Cl Negative ion to form ammonium chloride precipitate thus decreasing the conductivity by utilizing H positive ion to form water molecule due to this decrease in conductivity the graph shows on negative fall in conductance after all the edge positive and Cl negative ions are combine end point is reached where no free ions are possible for conduction after this when the basis added to form NH4 positive and was OH negative since it is a weak base it has low conductivity and the graph increases very slowly towards conduction minute changes in increasing conduction can be seen when a weak bases are added
plot a) show high conductivity which is not possible in weak bases
plot c) and d) involve 3 mixture components which is not the case
The perfect diagram would look like this and B is the closest.

plot c) and d) involve 3 mixture components which is not the case
The perfect diagram would look like this and B is the closest.


|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer?
Question Description
In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer?.
In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer?.
Solutions for In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer?, a detailed solution for In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In a typical Conductometric titration of a strong acid with a weak base, the curve resembles:a)b)c)d)Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















