Class 12 Exam > Class 12 Questions > can Dinitrogen trioxide be written as O--N-O-...
Start Learning for Free
can Dinitrogen trioxide be written as O--N-O-N--O
Most Upvoted Answer
can Dinitrogen trioxide be written as O--N-O-N--O
Introduction
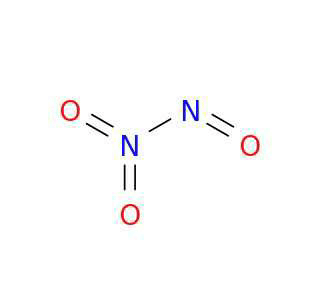
Dinitrogen trioxide (N2O3) is a chemical compound composed of two nitrogen atoms and three oxygen atoms. It is also known as nitrogen sesquioxide. In this response, we will explore the structure and bonding of dinitrogen trioxide and explain how it can be written as O--N--O--N--O.
Structure of Dinitrogen Trioxide
Dinitrogen trioxide is a linear molecule with a bent structure due to the lone pairs of electrons on the central nitrogen atom. The Lewis structure of N2O3 shows that the nitrogen atoms are connected by a double bond, and each nitrogen atom is bonded to an oxygen atom.
Writing Dinitrogen Trioxide as O--N--O--N--O
To represent the structure of dinitrogen trioxide as O--N--O--N--O, we need to consider the arrangement of atoms and the nature of the chemical bonds.
1. Oxygen-Nitrogen Single Bonds
In dinitrogen trioxide, there are two oxygen-nitrogen single bonds. Each oxygen atom is bonded to a nitrogen atom through a single bond. The representation O--N indicates a single bond between oxygen and nitrogen.
2. Nitrogen-Nitrogen Double Bond
The two nitrogen atoms in dinitrogen trioxide are connected by a double bond. The representation N--N indicates a double bond between the nitrogen atoms.
3. Oxygen Atoms
Each nitrogen atom in dinitrogen trioxide is also bonded to an oxygen atom. These oxygen atoms are represented as O.
Combining the Bonds
By combining the above representations, we can write dinitrogen trioxide as O--N--O--N--O. This indicates that the molecule consists of an oxygen atom bonded to a nitrogen atom, followed by a nitrogen-nitrogen double bond, then another oxygen atom bonded to the second nitrogen atom, and finally another oxygen atom.
Summary
Dinitrogen trioxide (N2O3) can be represented as O--N--O--N--O, indicating the arrangement of atoms and the nature of the chemical bonds. It consists of oxygen-nitrogen single bonds, a nitrogen-nitrogen double bond, and oxygen atoms bonded to nitrogen atoms. This representation helps visualize the structure of dinitrogen trioxide and understand its chemical properties.
Dinitrogen trioxide (N2O3) is a chemical compound composed of two nitrogen atoms and three oxygen atoms. It is also known as nitrogen sesquioxide. In this response, we will explore the structure and bonding of dinitrogen trioxide and explain how it can be written as O--N--O--N--O.
Structure of Dinitrogen Trioxide
Dinitrogen trioxide is a linear molecule with a bent structure due to the lone pairs of electrons on the central nitrogen atom. The Lewis structure of N2O3 shows that the nitrogen atoms are connected by a double bond, and each nitrogen atom is bonded to an oxygen atom.
Writing Dinitrogen Trioxide as O--N--O--N--O
To represent the structure of dinitrogen trioxide as O--N--O--N--O, we need to consider the arrangement of atoms and the nature of the chemical bonds.
1. Oxygen-Nitrogen Single Bonds
In dinitrogen trioxide, there are two oxygen-nitrogen single bonds. Each oxygen atom is bonded to a nitrogen atom through a single bond. The representation O--N indicates a single bond between oxygen and nitrogen.
2. Nitrogen-Nitrogen Double Bond
The two nitrogen atoms in dinitrogen trioxide are connected by a double bond. The representation N--N indicates a double bond between the nitrogen atoms.
3. Oxygen Atoms
Each nitrogen atom in dinitrogen trioxide is also bonded to an oxygen atom. These oxygen atoms are represented as O.
Combining the Bonds
By combining the above representations, we can write dinitrogen trioxide as O--N--O--N--O. This indicates that the molecule consists of an oxygen atom bonded to a nitrogen atom, followed by a nitrogen-nitrogen double bond, then another oxygen atom bonded to the second nitrogen atom, and finally another oxygen atom.
Summary
Dinitrogen trioxide (N2O3) can be represented as O--N--O--N--O, indicating the arrangement of atoms and the nature of the chemical bonds. It consists of oxygen-nitrogen single bonds, a nitrogen-nitrogen double bond, and oxygen atoms bonded to nitrogen atoms. This representation helps visualize the structure of dinitrogen trioxide and understand its chemical properties.
Community Answer
can Dinitrogen trioxide be written as O--N-O-N--O

|
Explore Courses for Class 12 exam
|

|
Question Description
can Dinitrogen trioxide be written as O--N-O-N--O for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about can Dinitrogen trioxide be written as O--N-O-N--O covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for can Dinitrogen trioxide be written as O--N-O-N--O.
can Dinitrogen trioxide be written as O--N-O-N--O for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about can Dinitrogen trioxide be written as O--N-O-N--O covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for can Dinitrogen trioxide be written as O--N-O-N--O.
Solutions for can Dinitrogen trioxide be written as O--N-O-N--O in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of can Dinitrogen trioxide be written as O--N-O-N--O defined & explained in the simplest way possible. Besides giving the explanation of
can Dinitrogen trioxide be written as O--N-O-N--O, a detailed solution for can Dinitrogen trioxide be written as O--N-O-N--O has been provided alongside types of can Dinitrogen trioxide be written as O--N-O-N--O theory, EduRev gives you an
ample number of questions to practice can Dinitrogen trioxide be written as O--N-O-N--O tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















