Chemistry Exam > Chemistry Questions > Which of the following statement(s) is/are co...
Start Learning for Free
Which of the following statement(s) is/are correct concerning redox properties ?
- a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent.
- b)The oxidizing power of halogens decreases from Chlorine to Iodine
- c)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.
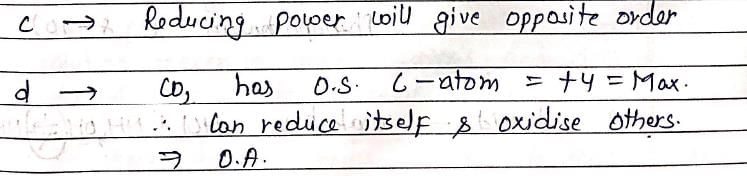
- d)CO2 is an oxidizing agent
Correct answer is option 'A,B,C,D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which of the following statement(s) is/are correct concerning redox pr...

Most Upvoted Answer
Which of the following statement(s) is/are correct concerning redox pr...
Redox properties explanation:
a) A metal M for which E0 for half-cell reaction Mn+ + ne- ⇌ M is very negative will be a good reducing agent.
- A metal with a very negative E0 value for the half-cell reaction will have a strong tendency to gain electrons, making it a good reducing agent.
b) The oxidizing power of halogens decreases from Chlorine to Iodine
- Halogens are elements in Group 17 of the periodic table, and their oxidizing power decreases as you move down the group. This means that Chlorine is a stronger oxidizing agent compared to Iodine.
c) The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.
- The reducing power of hydrogen halides increases as you move down the group from Hydrogen Chloride to Hydrogen Iodide. This is because the ability to donate electrons increases down the group.
d) CO2 is an oxidizing agent
- CO2 can act as an oxidizing agent in certain reactions where it accepts electrons. It is known to oxidize metals such as iron to form iron oxide.
Overall, all the statements are correct concerning redox properties as they explain the behavior of different substances in terms of their ability to gain or lose electrons during chemical reactions.
a) A metal M for which E0 for half-cell reaction Mn+ + ne- ⇌ M is very negative will be a good reducing agent.
- A metal with a very negative E0 value for the half-cell reaction will have a strong tendency to gain electrons, making it a good reducing agent.
b) The oxidizing power of halogens decreases from Chlorine to Iodine
- Halogens are elements in Group 17 of the periodic table, and their oxidizing power decreases as you move down the group. This means that Chlorine is a stronger oxidizing agent compared to Iodine.
c) The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.
- The reducing power of hydrogen halides increases as you move down the group from Hydrogen Chloride to Hydrogen Iodide. This is because the ability to donate electrons increases down the group.
d) CO2 is an oxidizing agent
- CO2 can act as an oxidizing agent in certain reactions where it accepts electrons. It is known to oxidize metals such as iron to form iron oxide.
Overall, all the statements are correct concerning redox properties as they explain the behavior of different substances in terms of their ability to gain or lose electrons during chemical reactions.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer?
Question Description
Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer?.
Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer?.
Solutions for Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer?, a detailed solution for Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer? has been provided alongside types of Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following statement(s) is/are correct concerning redox properties ?a)A metal M for which E0 for half-cell reaction Mn+ + ne– ↔ M is very negative will be a good reducing agent. b)The oxidizing power of halogens decreases from Chlorine to Iodinec)The reducing power of Hydrogen halides increases from Hydrogen Chloride to Hydrogen Iodide.d)CO2 is an oxidizing agentCorrect answer is option 'A,B,C,D'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















