Class 11 Exam > Class 11 Questions > Direction (Q. Nos. 10-11) This section contai...
Start Learning for Free
Direction (Q. Nos. 10-11) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. HF molecule also exist in liquid state. Types of intermolecular forces between them a
- a)intermolecular H-bonding
- b)intramolecular H-bonding
- c)dipole-dipole interaction
- d)London forces of attraction
Correct answer is option 'A,C'. Can you explain this answer?
Verified Answer
Direction (Q. Nos. 10-11) This section contains 4multiple choice quest...
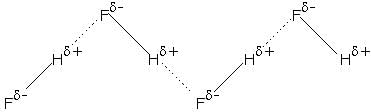
Here, both dipole-dipole interaction and intermolecular H-bonding takes place which makes HF a liquid.
Most Upvoted Answer
Direction (Q. Nos. 10-11) This section contains 4multiple choice quest...
**Explanation:**
The HF (hydrogen fluoride) molecule can exist in both gaseous and liquid states. In the liquid state, the intermolecular forces between HF molecules play a crucial role in determining the physical properties of the substance.
**a) Intermolecular H-bonding:**
The primary intermolecular force between HF molecules in the liquid state is hydrogen bonding. Hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom (such as fluorine, oxygen, or nitrogen) and interacts with the lone pairs of electrons on another highly electronegative atom. In the case of HF, the hydrogen atom is bonded to fluorine, and the lone pairs on the fluorine atom can form hydrogen bonds with neighboring HF molecules. This intermolecular force is relatively strong and contributes to the high boiling point and surface tension of liquid HF.
**c) Dipole-dipole interaction:**
In addition to hydrogen bonding, dipole-dipole interactions also play a role in the intermolecular forces between HF molecules in the liquid state. HF is a polar molecule, with the fluorine atom being more electronegative than the hydrogen atom. As a result, there is an uneven distribution of electron density, creating a dipole moment. The positive end of one HF molecule is attracted to the negative end of another HF molecule, leading to dipole-dipole interactions. These interactions are weaker than hydrogen bonding but still contribute to the overall intermolecular forces in liquid HF.
**b) Intramolecular H-bonding:**
Intramolecular hydrogen bonding refers to hydrogen bonding that occurs within a single molecule. In the case of HF, intramolecular hydrogen bonding is not relevant as there is only one HF molecule involved.
**d) London forces of attraction:**
London dispersion forces, also known as London forces or van der Waals forces, are weak intermolecular forces that occur between all molecules, including HF. These forces arise from temporary fluctuations in electron distribution, leading to momentary dipoles. While London forces are present in HF, they are weaker than hydrogen bonding and dipole-dipole interactions in determining the properties of liquid HF.
In summary, the intermolecular forces between HF molecules in the liquid state include intermolecular hydrogen bonding (a) and dipole-dipole interactions (c).
The HF (hydrogen fluoride) molecule can exist in both gaseous and liquid states. In the liquid state, the intermolecular forces between HF molecules play a crucial role in determining the physical properties of the substance.
**a) Intermolecular H-bonding:**
The primary intermolecular force between HF molecules in the liquid state is hydrogen bonding. Hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom (such as fluorine, oxygen, or nitrogen) and interacts with the lone pairs of electrons on another highly electronegative atom. In the case of HF, the hydrogen atom is bonded to fluorine, and the lone pairs on the fluorine atom can form hydrogen bonds with neighboring HF molecules. This intermolecular force is relatively strong and contributes to the high boiling point and surface tension of liquid HF.
**c) Dipole-dipole interaction:**
In addition to hydrogen bonding, dipole-dipole interactions also play a role in the intermolecular forces between HF molecules in the liquid state. HF is a polar molecule, with the fluorine atom being more electronegative than the hydrogen atom. As a result, there is an uneven distribution of electron density, creating a dipole moment. The positive end of one HF molecule is attracted to the negative end of another HF molecule, leading to dipole-dipole interactions. These interactions are weaker than hydrogen bonding but still contribute to the overall intermolecular forces in liquid HF.
**b) Intramolecular H-bonding:**
Intramolecular hydrogen bonding refers to hydrogen bonding that occurs within a single molecule. In the case of HF, intramolecular hydrogen bonding is not relevant as there is only one HF molecule involved.
**d) London forces of attraction:**
London dispersion forces, also known as London forces or van der Waals forces, are weak intermolecular forces that occur between all molecules, including HF. These forces arise from temporary fluctuations in electron distribution, leading to momentary dipoles. While London forces are present in HF, they are weaker than hydrogen bonding and dipole-dipole interactions in determining the properties of liquid HF.
In summary, the intermolecular forces between HF molecules in the liquid state include intermolecular hydrogen bonding (a) and dipole-dipole interactions (c).

|
Explore Courses for Class 11 exam
|

|
Question Description
Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer?.
Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer?.
Solutions for Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer?, a detailed solution for Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer? has been provided alongside types of Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Direction (Q. Nos. 10-11) This section contains 4multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of whichONE or MORE THANT ONE is correct.Q.HF molecule also exist in liquid state. Types of intermolecular forces between them aa)intermolecular H-bondingb)intramolecular H-bondingc)dipole-dipole interactiond)London forces of attractionCorrect answer is option 'A,C'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















